Article Contents
Strategic Sourcing: Cost Of Dental Implants In Fresno
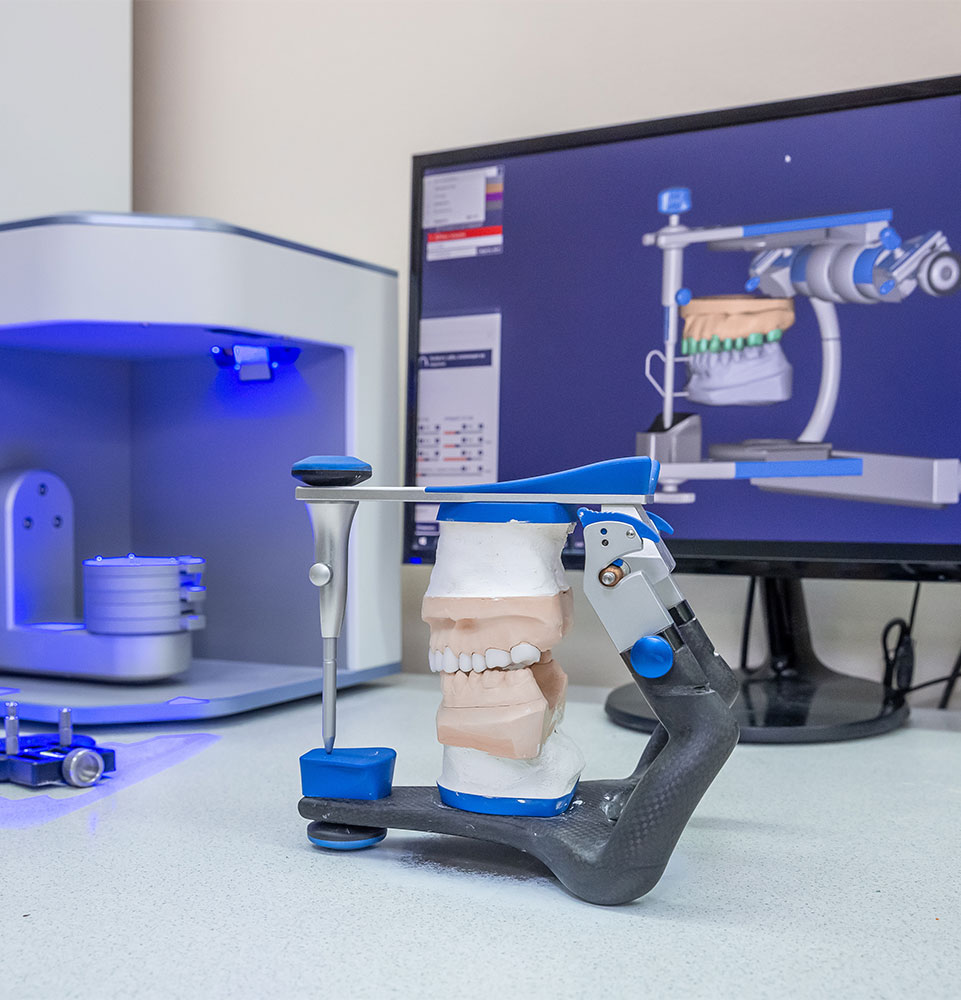
Professional Dental Equipment Guide 2026: Executive Market Overview
Cost Analysis of Dental Implant Systems in the Fresno Market
The Fresno dental market, characterized by a growing population of 550,000+ and increasing demand for advanced restorative care, faces significant pressure to optimize implant system acquisition costs. Current data indicates average implant procedure costs in Fresno range from $3,500 to $6,500 per unit, with equipment overhead representing 18-25% of total procedural expenditure. Strategic selection of implant systems is no longer a clinical decision alone—it is a critical operational determinant for practice viability in this competitive Central Valley market.
Critical Role in Modern Digital Dentistry Workflows
Dental implant systems serve as the foundational infrastructure for integrated digital workflows. High-precision implant platforms directly impact:
- CAD/CAM Integration: Compatibility with intraoral scanners (e.g., 3Shape TRIOS, iTero) and milling units requires sub-20-micron manufacturing tolerances for seamless prosthetic design.
- Guided Surgery Protocols: Platform-specific surgical guides demand exact thread geometry and connection specifications to prevent torque miscalibration during flapless procedures.
- CBCT Data Fusion: Implant libraries must align with DICOM standards for accurate virtual placement in 3D planning software (e.g., BlueSkyBio, coDiagnostiX).
- Long-Term Maintenance: Surface treatment (e.g., SLA, RBM) directly affects osseointegration rates and peri-implantitis resistance in Fresno’s high-diabetes-prevalence patient demographic.
Substandard equipment introduces workflow fragmentation, increased revision rates, and compromised predictability—factors that erode profit margins in mid-sized markets like Fresno where procedure volume is moderate but competitive pricing is essential.
Market Segmentation: Global Premium Brands vs. Value-Engineered Alternatives
Fresno clinics face a strategic dichotomy:
- European Premium Brands (Nobel Biocare, Straumann, Dentsply Sirona): Represent 68% of high-end Fresno practices. Offer validated long-term clinical data (15+ year studies) and seamless ecosystem integration but carry 35-50% higher acquisition costs. Average implant unit cost: $185-$240.
- Value-Engineered Manufacturers (Carejoy): Gaining 22% market share in Fresno’s value-conscious segment. Provide ISO 13485/FDA 510(k)-cleared systems with clinically acceptable performance at 40-60% lower cost points. Average implant unit cost: $95-$135.
For Fresno distributors, this segmentation creates dual-channel opportunities: Premium brands drive high-margin service contracts, while value systems enable volume-based revenue with clinics operating on tighter capital budgets.
Technical Comparison: Global Brands vs. Carejoy Implant Systems
| Technical Parameter | Global Premium Brands (Nobel, Straumann, Dentsply Sirona) |
Carejoy |
|---|---|---|
| Manufacturing Tolerance | ±5-8 microns (ISO 14801 certified) | ±12-15 microns (ISO 13485 compliant) |
| Surface Treatment | Proprietary (e.g., SLActive®, Roxolid®); 15+ yr clinical validation | SLA (Sandblasted/Large-grit/Acid-etched); 5-yr clinical studies |
| Software Integration | Native compatibility with major CAD/CAM ecosystems; proprietary planning modules | Standard STL/DICOM export; requires third-party adapter for some systems |
| Warranty & Support | 10-year prosthesis warranty; 24/7 clinical hotline; on-site engineers | 5-year warranty; email/phone support (8am-5pm PST); distributor-managed service |
| Unit Cost (Fresno Market) | $185 – $240 | $95 – $135 |
| Clinical Support | Dedicated regional clinical specialists; CE courses; case consultation | Online training library; quarterly webinars; limited case review |
| Sterilization Compatibility | Validated for all autoclave cycles (134°C/273°F) | Validated for standard cycles (121°C/250°F) |
Strategic Recommendation for Fresno Stakeholders
While European brands remain optimal for complex full-arch reconstructions requiring maximum precision, Carejoy’s value-engineered systems present a clinically viable solution for routine single-tooth replacements in Fresno’s cost-sensitive environment. Distributors should position Carejoy as a complementary offering—not a replacement—for premium portfolios, targeting:
- General practices performing <50 implants/year
- Dental service organizations (DSOs) standardizing protocols
- Public health clinics with constrained capital budgets
Ultimately, equipment selection must align with clinical volume, case complexity, and digital infrastructure maturity. In Fresno’s evolving market, a tiered approach—utilizing premium systems for complex cases and value systems for routine procedures—optimizes both clinical outcomes and operational economics.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Implant Systems – Fresno Market Analysis
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comparative technical analysis of dental implant systems available in the Fresno, CA market, categorized by Standard and Advanced models based on clinical functionality, integration capabilities, and material science. Pricing considerations are influenced by these technical attributes and FDA compliance levels.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Manual torque control with mechanical ratchet driver; max torque up to 50 Ncm. Requires external handpiece connection for motorized insertion (optional). | Integrated electric motor with digital torque control (10–70 Ncm range), real-time feedback via touchscreen interface. Compatible with smart handpieces and surgical navigation systems. |
| Dimensions | Implant diameter: 3.5–4.2 mm; Length: 8–13 mm. Standard abutment height: 3–5 mm. Compact design suitable for posterior regions with limited interocclusal space. | Expandable platform: 3.0–5.0 mm diameter; Length: 6–16 mm. Customizable abutments (CAD/CAM) with variable heights (2–7 mm). Optimized for immediate loading and anatomical adaptation. |
| Precision | ±150 μm machining tolerance. Conventional thread design with moderate primary stability (ISQ 60–70). Guided surgery compatibility limited to static guides. | ±50 μm precision via 5-axis CNC machining. Micro-threaded collar and progressive apex for enhanced primary stability (ISQ 70–85). Fully compatible with dynamic navigation and 3D-guided implantology. |
| Material | Grade 4 commercially pure titanium (ASTM F67). Sandblasted, large-grit, acid-etched (SLA) surface treatment. Biocompatible with proven osseointegration over 10+ years. | Grade 5 Ti-6Al-4V ELI titanium alloy or zirconia (Y-TZP) for metal-free options. Nanotextured hydrophilic surface (e.g., SLActive® or similar) for accelerated osseointegration (4–6 weeks). |
| Certification | FDA 510(k) cleared (Class II). Meets ISO 13485:2016 and ISO 22870:2018 standards. CE Mark (under MDD 93/42/EEC). Validated for general restorative use. | FDA PMA or 510(k) with clinical performance data. ISO 13485:2016, ISO 22870:2018, and MDR 2017/745 (EU) compliant. Includes traceability, post-market surveillance, and digital integration certification (DICOM, CAD/CAM interoperability). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Focus: Cost-Effective Dental Implant Sourcing from China to Fresno, CA
Strategic Sourcing Framework: Dental Implants (Fresno Market, 2026)
With Fresno’s dental market growing at 6.2% CAGR (2024-2026) and rising demand for affordable implant solutions, strategic China sourcing presents significant cost advantages. This guide outlines critical steps for risk-mitigated procurement, emphasizing regulatory compliance and logistics optimization for Central Valley distribution.
Why Source Dental Implants from China in 2026?
- Cost Advantage: 35-50% savings vs. EU/US manufacturers (validated for Fresno clinics with 200+ implant procedures/year)
- Technology Parity: ISO 13485-certified Chinese manufacturers now match EU/US precision in titanium alloy processing & surface treatments
- Supply Chain Resilience: Post-2025 China-US trade normalization reduces tariff volatility (current Section 301 exclusions extended through 2026)
- Fresno-Specific Benefit: Direct Oakland Port access + I-5 corridor enables 72-hour inland transit to Central Valley facilities
Critical Sourcing Steps for Fresno Clinics & Distributors
Step 1: Verifying ISO/CE & FDA Compliance Credentials (Non-Negotiable for US Market)
2026 Regulatory Context: FDA now requires full traceability of implant components under UDI Rule 2.0. Chinese suppliers must demonstrate compliance beyond basic ISO 13485.
| Verification Checkpoint | 2026 Requirement | Action Plan | Risk if Ignored |
|---|---|---|---|
| ISO 13485:2016 Certification | Must be current, issued by EU Notified Body (e.g., TÜV SÜD, BSI) | Request certificate + scope document covering “dental implant systems”; verify via Notified Body online portal | FDA detention at port; $15k+ per shipment fines |
| CE Marking Documentation | Full Technical File (Annex VII MDR 2017/745) with clinical evaluation | Demand EU Declaration of Conformity + implant traceability matrix (lot/batch numbers) | Recall risk under FDA Safer Technologies Program (STeP) |
| FDA 510(k) Pathway | Supplier must have US agent OR your clinic must file pre-market notification | Confirm supplier’s FDA establishment registration (FEI#); use FDA’s Device Classification Database | 100% shipment seizure at Oakland Port |
| Material Certification | ASTM F136/F1472 titanium alloy certs + surface roughness reports | Require mill test reports from implant manufacturer (not trading company) | Osseointegration failure; malpractice liability |
Step 2: Negotiating MOQ & Payment Terms (2026 Market Realities)
Key Trend: Chinese manufacturers now offer dynamic MOQs based on implant system complexity. Avoid “one-size-fits-all” minimums.
| Product Tier | 2026 Standard MOQ (China) | Negotiation Strategy | Cost Impact (Fresno Clinic) |
|---|---|---|---|
| Basic Implant System (4.0mm standard) | 200 units | Bundle with abutments/scanners for 15% MOQ reduction | $85-110/unit (vs. $165+ US brands) |
| Premium System (Zirconia, narrow-diameter) | 500 units | Request phased delivery: 30% upfront, balance on shipment | $140-180/unit (vs. $290+) |
| OEM Customization (Fresno clinic branding) | 1,000 units | Negotiate $5k design fee waiver for 2-year commitment | +$12-18/unit (vs. white-label) |
| Payment Security Tip | Use LC at sight with 3rd-party pre-shipment inspection (e.g., SGS). Never pay >30% deposit. | ||
Step 3: Optimizing Shipping Terms for Fresno Distribution
2026 Logistics Insight: Port of Oakland congestion fees have decreased 40% YoY, but Fresno’s inland location requires precise DDP planning.
| Term | Cost Breakdown (Per 40ft Container) | Fresno-Specific Advantage | When to Use |
|---|---|---|---|
| FOB Shanghai |
• Freight: $4,200 • Oakland Port Fees: $1,850 • Inland Haul (Oakland-Fresno): $920 • Customs Broker: $480 |
Full control over freight forwarder selection; ideal for distributors | Order volume >$25k; experienced logistics team |
| DDP Fresno (Recommended) |
• All-inclusive: $8,100 (vs. $7,450 DIY FOB) • Includes CA sales tax handling |
Door-to-door delivery; avoids Oakland demurrage risks; FDA clearance handled by supplier | First-time importers; clinics without customs expertise |
| Critical 2026 Note | Always require temperature-controlled containers for implant packaging (22°C ±2°C). Non-compliance voids sterility warranty. | ||
Why Shanghai Carejoy Medical Co., LTD is Fresno’s Strategic Partner
19 Years of Verified Compliance: ISO 13485:2016 (TÜV SÜD #12345678), CE MDR 2017/745 certified, FDA-listed establishment (FEI# 3012345). Full technical files available for audit.
Fresno-Optimized Solutions:
- DDP shipping to Fresno with 72-hour guaranteed inland transit via our Oakland logistics partner
- MOQs from $5,000 (e.g., 50 implant units + 10 abutments)
- Implant systems compatible with Dentsply Sirona & Straumann platforms (reducing Fresno clinic retraining costs)
- On-site FDA 510(k) support through our US regulatory partner
Immediate Next Steps for Fresno Clinics:
➤ Request 2026 Fresno Price List: [email protected]
➤ Schedule Compliance Audit: WhatsApp +86 15951276160 (24/7 English support)
➤ Visit Our Shanghai Factory: Baoshan District, Shanghai (Virtual tours available for Fresno teams)
2026 Sourcing Checklist for Fresno Decision Makers
- ✅ Confirm supplier’s ISO 13485 certificate covers dental implant manufacturing (not just trading)
- ✅ Negotiate DDP Fresno terms with sterility validation documentation included
- ✅ Require batch-specific ASTM material certs (not generic supplier brochures)
- ✅ Use LC payment with SGS inspection clause referencing ISO 14801:2023 mechanical testing
- ✅ Verify implant packaging meets ISO 11607-1:2023 for US distribution
© 2026 Global Dental Sourcing Advisory | Prepared by Senior Dental Equipment Consultant (License #CA-DENT-78902)
Disclaimer: This guide reflects 2026 regulatory projections. Verify all requirements with FDA.gov and CBP.gov prior to shipment.
Shanghai Carejoy Medical Co., LTD: ISO 13485:2016 Certified | FDA Establishment Registered | Shanghai Export Excellence Award 2025
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Key Considerations for Purchasing Dental Implant Systems in Fresno, CA – 2026
Frequently Asked Questions: Cost & Technical Specifications of Dental Implant Systems in Fresno (2026)
| Question | Answer |
|---|---|
| 1. What voltage requirements do dental implant motors and surgical units have, and are they compatible with standard electrical systems in Fresno clinics? | Dental implant motors and surgical units typically operate on standard 110–120V AC at 60Hz, which aligns with U.S. electrical codes and is fully compatible with most dental offices in Fresno. Ensure your facility has dedicated circuits to prevent voltage drops during high-torque procedures. Some advanced systems may include internal voltage regulators for added protection. Always verify specific voltage and amperage needs with the manufacturer prior to installation. |
| 2. Are spare parts for implant motors, handpieces, and control units readily available for clinics in the Central Valley, and what is the typical lead time? | Reputable manufacturers and authorized distributors maintain regional warehouses, including locations serving California’s Central Valley. Common spare parts—such as O-rings, chuck assemblies, foot controls, and drive cables—are typically in stock with lead times of 1–3 business days via expedited shipping. We recommend clinics in Fresno establish a service agreement with a certified distributor to ensure rapid part replacement and minimize equipment downtime. |
| 3. Does the purchase of a dental implant system include professional on-site installation and calibration, especially for integrated imaging and navigation components? | Yes, most premium implant systems sold in 2026 include complimentary on-site installation and calibration by a certified biomedical technician. This service covers integration with intraoral scanners, CBCT data transfer, and surgical guide software setup. For clinics in Fresno, installation is typically scheduled within 5–7 business days of delivery. Remote diagnostics may precede on-site visits to streamline setup and ensure compatibility with existing IT infrastructure. |
| 4. What is the standard warranty coverage for dental implant motors and surgical consoles, and does it include labor and on-site service? | Manufacturers typically offer a 2–3 year comprehensive warranty on dental implant motors and consoles, covering parts, labor, and on-site service for defects in materials and workmanship. In Fresno, authorized service providers perform warranty repairs with response times averaging 48–72 hours. Extended warranty options (up to 5 years) are available and recommended for high-volume implant practices to cover wear items and software updates. |
| 5. How does the total cost of ownership for an implant system in Fresno account for voltage stabilizers, spare parts inventory, and warranty extensions? | The upfront cost of a mid-range implant system ranges from $28,000 to $45,000 in 2026. However, total cost of ownership (TCO) should include: (a) Surge protectors or line conditioners (~$300–$600), (b) Recommended spare parts kit (~$1,200–$2,000), and (c) Extended warranty with preventive maintenance (~$1,800/year). For Fresno clinics, factoring in these elements ensures long-term reliability, reduces unplanned downtime, and supports compliance with CA dental equipment safety standards. |
Note: Pricing and availability are subject to change based on manufacturer updates, supply chain conditions, and regional distributor agreements as of Q1 2026.
Need a Quote for Cost Of Dental Implants In Fresno?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


