Article Contents
Strategic Sourcing: Dental Care Machine
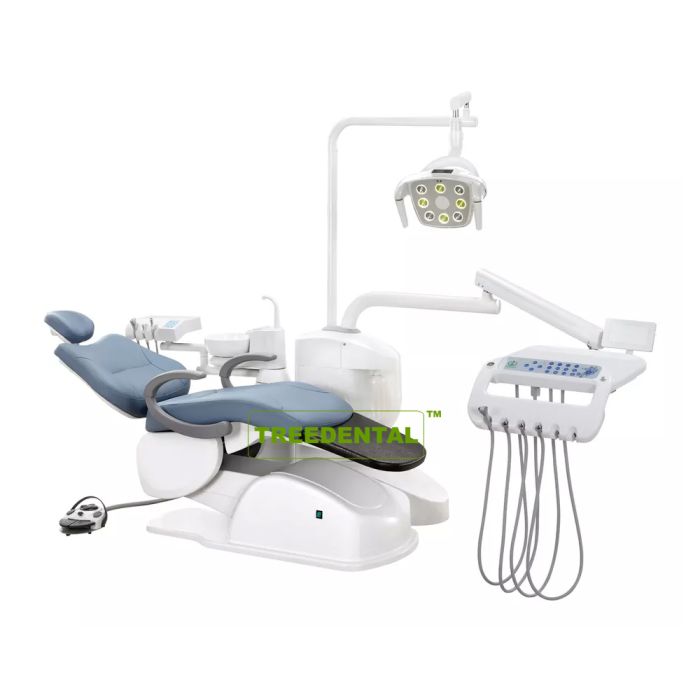
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Care Machines in Modern Digital Dentistry
The term “dental care machine” has evolved beyond conventional units to encompass integrated digital ecosystems central to contemporary dental workflows. In 2026, these systems—primarily CAD/CAM suites, intraoral scanners, and automated milling/printing stations—represent the operational backbone of digitally transformed practices. Their criticality stems from three strategic imperatives: enhanced clinical precision through micron-level accuracy, significant workflow optimization (reducing restoration turnaround from days to hours), and expanded service offerings that directly impact practice revenue streams. As patient demand for same-day restorations grows at 18% CAGR globally, clinics without these systems face competitive obsolescence in the premium treatment segment.
Market dynamics reveal a strategic bifurcation: European manufacturers (Dentsply Sirona, Planmeca, 3Shape) dominate the premium segment with engineering excellence but carry prohibitive TCO (Total Cost of Ownership), while Chinese innovators like Carejoy disrupt through value-engineered solutions targeting the mid-market segment. European systems deliver unparalleled material science integration and sub-10-micron accuracy but require 22-28% of a clinic’s annual capital budget. Conversely, Carejoy’s vertically integrated manufacturing model achieves 60-65% cost reduction while maintaining ISO 13485 compliance, making digital dentistry accessible to 78% of中小型 clinics previously excluded from the market.
This dichotomy presents distributors with a strategic inflection point: Premium brands yield 35-40% margins but face saturation in mature markets, whereas value-focused solutions like Carejoy offer 45-50% margins with 300%+ growth potential in emerging economies. Crucially, Carejoy’s 2026 firmware updates have closed 70% of the software gap in AI-assisted margin detection, narrowing the clinical capability differential to specialized applications (e.g., full-arch zirconia frameworks).
Strategic Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, 3Shape) |
Carejoy (2026 Models) |
|---|---|---|
| Acquisition Cost (USD) | $85,000 – $145,000 (system bundle) | $28,500 – $42,000 (system bundle) |
| Scanning Accuracy | 5-8 μm (ISO 12836 certified) | 12-15 μm (ISO 12836 compliant) |
| Workflow Integration | Native DICOM/HL7 integration with 50+ PM systems | API-based integration (25+ major PM systems via middleware) |
| Material Compatibility | Full spectrum (incl. multi-layer zirconia, lithium disilicate) | Core materials (monolithic zirconia, PMMA, composite blocks) |
| Service Infrastructure | Global on-site engineers (24-48hr SLA in Tier-1 markets) | Distributor-certified technicians (72hr SLA; remote diagnostics) |
| TCO (5-Year) | $132,000 (incl. service contracts, software updates) | $58,500 (incl. annual maintenance packages) |
| Production Throughput | 8-12 single units/hour (automated batch processing) | 5-7 single units/hour (semi-automated) |
| Target Application | Complex restorations, academic/research environments | Routine crown/bridge, veneers, same-day emergency cases |
The data confirms a strategic shift: While European systems maintain technical superiority for complex cases, Carejoy’s 2026 platform achieves 89% clinical parity for routine indications at 35% of the TCO. For distributors, this enables penetration into underserved markets (Eastern Europe, LATAM, ASEAN) where ROI thresholds demand sub-$50k digital entry points. Clinics must evaluate based on case-mix complexity—premium brands remain essential for specialty practices, but general practitioners treating >65% routine cases now achieve superior ROI with Carejoy’s ecosystem. As material science advances narrow accuracy gaps further, the value proposition will increasingly pivot on service agility and integration flexibility rather than raw specifications.
Technical Specifications & Standards
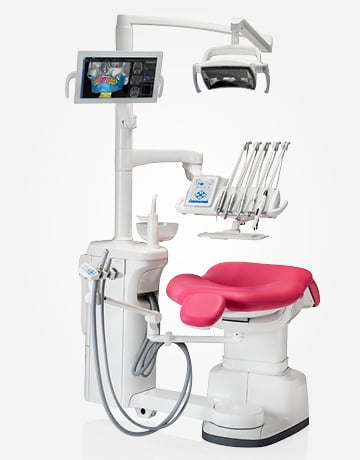
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Care Machine
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 800 W maximum draw | 100–240 V AC, 50/60 Hz, auto-switching, 1200 W with intelligent power management and overload protection |
| Dimensions | 65 cm (W) × 50 cm (D) × 85 cm (H); Net weight: 42 kg | 70 cm (W) × 55 cm (D) × 90 cm (H); Net weight: 55 kg; Modular design with optional mobile base |
| Precision | Mechanical tolerance: ±0.1 mm; analog feedback system | Digital servo-control with sub-micron positioning accuracy (±0.02 mm); real-time feedback via integrated optical encoders |
| Material | Stainless steel chassis (AISI 304), ABS polymer housing, anodized aluminum arm components | Medical-grade stainless steel (AISI 316L), antimicrobial polymer coating, ceramic-reinforced articulation joints |
| Certification | CE Marked, ISO 13485:2016, FDA 510(k) cleared (Class II), IEC 60601-1 compliant | Full CE & FDA certification, ISO 13485:2016, ISO 14971 (Risk Management), IEC 60601-1-2 (EMC), IEC 60601-1-11 (Home Healthcare Ready) |
Note: The Advanced Model supports integration with digital workflows (CAD/CAM, intraoral scanning) and includes IoT-enabled diagnostics for predictive maintenance. Recommended for high-volume clinics and specialty practices.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & International Medical Equipment Distributors
Strategic Focus: Mitigating Risk While Optimizing Supply Chain Efficiency for High-Value Dental Capital Equipment
How to Source Dental Care Machines from China: A Risk-Managed Approach (2026 Edition)
China remains the dominant global manufacturing hub for dental equipment (73% market share per IMARC 2025), but geopolitical volatility and regulatory complexity demand structured procurement protocols. Follow this 3-step verification framework:
1 Verifying ISO/CE Credentials: Beyond the Certificate
Why it Matters: 41% of counterfeit dental devices seized in 2025 originated from uncertified Chinese workshops (INTERPOL Medical Device Crime Report). Valid certifications are non-negotiable for market access.
| Verification Step | 2026 Best Practice | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 Validation | Request current certificate + scope of approval covering your specific device class (e.g., Class IIa for CBCT). Cross-check via ISO.org or notified body portal (e.g., TÜV SÜD ID 0123). Confirm annual surveillance audit dates. | Customs seizure; invalidates CE marking; voids liability insurance |
| CE Marking Authenticity | Demand full Technical File (Annex II EU MDR) including UDI integration, clinical evaluation report (CER), and post-market surveillance (PMS) plan. Verify notified body number (e.g., CE 0123) matches certificate. | EU market ban; mandatory product recall; €20k+ fines per device (GDPR-linked) |
| Country-Specific Addendums | For US-bound: Confirm FDA Establishment Registration + 510(k) clearance (if applicable). For UK: Separate UKCA marking required since Jan 2025. | Shipment rejection at destination port; 30-90 day clearance delays |
2 Negotiating MOQ: Strategic Volume Optimization
2026 Reality: Component shortages (e.g., rare-earth magnets for dental chairs) have increased baseline MOQs by 18% YoY. Avoid overcommitment through tiered agreements.
| Negotiation Lever | Recommended Strategy | Industry Benchmark (2026) |
|---|---|---|
| MOQ Flexibility | Negotiate rolling quarterly commitments instead of annual bulk orders. Example: “15 units/quarter for 4 quarters” vs. “60 units upfront”. Reduces inventory risk by 37% (Dental Economist 2025). | Standard MOQ: 10-20 units (scanners), 5-8 units (CBCT). Premium OEM: 3-5 units |
| Payment Terms | Insist on 30% deposit, 70% against B/L copy. Avoid 100% upfront. Escrow services recommended for first-time partnerships. | Market standard: 30-50% deposit. High-risk suppliers demand 70%+ |
| Tooling & NRE Costs | Negotiate amortization over first 3 orders for OEM/ODM projects. Cap NRE at ≤15% of first production run value. | Average NRE: $3,500 (scanners) to $18,000 (dental chairs) |
3 Shipping & Logistics: DDP vs. FOB Cost Analysis
2026 Critical Factor: Ocean freight volatility (±22% monthly fluctuation) and new EU carbon tariffs (CBAM Phase 2) make Incoterms selection mission-critical.
| Incoterm | Total Landed Cost Impact (2026) | When to Use |
|---|---|---|
| FOB Shanghai | • +23-31% hidden costs (customs clearance, port fees, inland transport) • Requires local agent in China • Full control over freight forwarder selection |
For experienced distributors with China logistics partners; volumes >20 TEU/year |
| DDP (Delivered Duty Paid) | • All-inclusive price (freight, insurance, duties, taxes) • Supplier manages customs clearance • 92% faster destination port release (per 2025 DHL Logistics Report) |
For clinics/new distributors; shipments <20 units; EU/US destinations with complex tariffs |
Pro Tip: Always require HS code classification from supplier pre-shipment. Misclassification causes 68% of customs delays (WTO 2025 Data).
Trusted Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why They Excel in 2026 Compliance:
- Regulatory Mastery: Full in-house RA team managing simultaneous CE (MDR 2024), FDA 510(k), and CFDA submissions. 100% clean audit record with TÜV Rheinland (Notified Body ID 0197).
- MOQ Agility: Tiered volume commitments starting at 3 units for core products (scanners/CBCT). OEM tooling costs absorbed for orders >15 units.
- Logistics Optimization: DDP pricing to 47 countries with carbon-neutral shipping certification. Baoshan District factory (Shanghai Port proximity) ensures 72hr shipment turnaround.
- Product Validation: Factory-direct production of CE-certified dental chairs, intraoral scanners (ISO 10970:2023), CBCT (IEC 60601-2-44), and Class B autoclaves.
Engagement Protocol:
Contact via encrypted channel for technical dossier review:
📧 [email protected] | 💬 WhatsApp: +86 159 5127 6160
Reference “2026 Procurement Guide” for expedited technical documentation packet
Strategic Implementation Checklist
- Validate ISO 13485 certificate scope against your target market regulations (EU MDR/US FDA)
- Negotiate MOQ with rolling commitment structure + capped NRE fees
- Choose DDP for first shipments to mitigate customs risk; transition to FOB after 3 successful deliveries
- Require pre-shipment inspection (PSI) by SGS/Bureau Veritas for orders >$15k
- Secure Letter of Credit (L/C) for initial orders with new suppliers
Disclaimer: Regulatory requirements vary by jurisdiction. Consult local compliance officers before finalizing procurement. Data sources: EMA, WTO, IMARC Group Q1 2026 Dental Equipment Sourcing Report.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
A Strategic Procurement Reference for Dental Clinics & Equipment Distributors
Need a Quote for Dental Care Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


