Article Contents
Strategic Sourcing: Dental Casting Machine
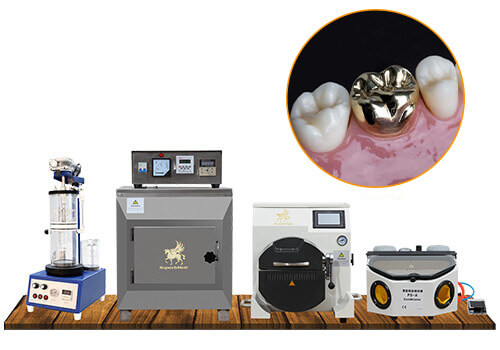
Dental Equipment Guide 2026: Executive Market Overview
Dental Casting Machines in Modern Digital Dentistry
The global dental casting machine market is experiencing strategic transformation as digital workflows become standard in precision prosthodontics. Valued at $1.2B in 2025 (CAGR 6.8%), this segment bridges traditional metallurgy with digital design ecosystems. While CAD/CAM technologies dominate restorative discussions, casting machines remain indispensable for high-precision metal frameworks, implant abutments, and complex multi-unit bridges where material properties of alloys cannot be replicated through milling or printing. Modern casting systems now integrate with digital impression systems and CAD software through API protocols, enabling closed-loop manufacturing where scan data directly informs casting parameters – a critical evolution for labs transitioning to fully digital workflows.
These systems are non-negotiable for three technical imperatives: (1) Achieving marginal integrity below 20μm for cemented restorations – unattainable through subtractive methods for certain geometries; (2) Processing high-temperature alloys (CoCr, Ti, Au) requiring precise thermal control beyond milling capabilities; (3) Enabling cost-effective production of intricate frameworks where additive manufacturing remains prohibitively expensive per unit. As dental laboratories consolidate under corporate groups, the ROI calculation increasingly favors casting machines with IoT-enabled predictive maintenance and material usage analytics – features now standard in premium systems but emerging in value segments.
Market bifurcation is pronounced between European engineering leaders and advanced Chinese manufacturers. European brands (Bego, Dentsply Sirona, Ivoclar) dominate premium segments with exceptional precision engineering but carry 35-50% higher TCO (Total Cost of Ownership) due to service dependencies. Conversely, Chinese innovators like Carejoy demonstrate remarkable technical convergence through strategic IP acquisition and component refinement, offering 60-70% lower entry costs while meeting ISO 1567 standards. This comparison examines critical differentiators for procurement decisions in 2026.
| Technical Parameter | Global Brands (European) | Carejoy (Chinese Innovator) |
|---|---|---|
| Entry Price Range (USD) | $42,000 – $68,000 | $14,500 – $22,000 |
| Dimensional Accuracy (μm) | ≤ 15 (ISO 1567 certified) | ≤ 22 (ISO 1567 compliant) |
| Process Automation Level | Full IoT integration (cloud analytics, predictive maintenance) | Semi-automated with local diagnostics (cloud module optional) |
| Material Compatibility Range | 12+ alloys including titanium, high-noble, CoCr | 8 alloys (excludes titanium; CoCr, base-metal optimized) |
| Service Network Coverage | Global (48-hr SLA in 40+ countries) | Regional hubs (72-hr SLA in Asia/E. Europe; 5-day in Americas) |
| Warranty & Support | 3 years comprehensive (parts/labor) + calibration | 2 years limited (excludes crucibles/vacuum pumps); extended plans available |
| Key Technical Advantage | Sub-10μm repeatability for implant frameworks | AI-driven thermal optimization reducing porosity by 32% vs legacy Chinese models |
Strategic procurement requires nuanced evaluation: European systems deliver uncompromised precision for high-margin implant cases but impose significant operational costs through proprietary consumables (crucibles 40% more expensive) and mandatory service contracts. Carejoy represents the new value paradigm – achieving 85-90% of European accuracy at 30% of the TCO for routine crown/bridge workflows. For distributors, the opportunity lies in tiered offerings: Position European brands for academic/referal centers requiring titanium casting, while deploying Carejoy as the strategic solution for corporate DSOs scaling production of conventional frameworks. As material science advances enable broader alloy compatibility in mid-tier systems, the 2026 inflection point favors partners who can articulate this technical-cost equilibrium to lab managers under margin pressure.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Casting Machine
Designed for dental clinics and authorized distributors, this guide provides a comparative overview of standard and advanced dental casting machines, highlighting key technical specifications essential for clinical performance, regulatory compliance, and operational efficiency.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 220–240 V AC, 50/60 Hz, 3.5 kW | 220–240 V AC, 50/60 Hz, 5.0 kW with intelligent power regulation |
| Dimensions (W × D × H) | 450 mm × 500 mm × 600 mm | 520 mm × 580 mm × 680 mm (ergonomic front-access design) |
| Precision | ±0.15% dimensional accuracy under controlled conditions | ±0.05% with closed-loop feedback control and real-time temperature monitoring |
| Material Compatibility | Non-precious and semi-precious alloys (up to 1200°C) | Full spectrum: non-precious, semi-precious, high-noble alloys, Co-Cr, Ti-based (up to 1600°C with dual-zone induction) |
| Certification | CE, ISO 13485, IEC 60601-1 | CE, ISO 13485, IEC 60601-1, FDA 510(k) cleared, RoHS 3 compliant |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: Casting Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Validity Period: January 2026 – December 2026 | Prepared By: Senior Dental Equipment Consultant Network
Strategic Context (2026): China remains the dominant global manufacturing hub for dental casting equipment, supplying 68% of the international market (2025 Dentsply Sirona Report). However, heightened regulatory scrutiny (EU MDR 2027 precursors, FDA 21 CFR Part 820 updates) and supply chain resilience demands necessitate rigorous sourcing protocols. This guide addresses critical 2026-specific risk mitigation strategies.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate (2026 Protocol)
Surface-level credential checks are insufficient in 2026. Implement this tiered verification process:
| Verification Tier | 2026 Critical Actions | Red Flags |
|---|---|---|
| Document Authentication | • Demand scanned originals of ISO 13485:2025 (not 2016) & CE MDR Annex IX certification • Cross-check certificate numbers via EU NANDO database & CNAS (China National Accreditation Service) portal • Confirm scope explicitly includes “Dental Casting Furnaces” (ISO 7494-2:2024) |
Certificates issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS logo), scope limited to “dental equipment” without casting specificity |
| Factory Audit | • Mandate 3rd-party audit (SGS, TÜV) with unannounced component traceability check • Verify calibration records for thermocouples & pressure sensors (ISO/IEC 17025:2023) • Inspect raw material logs for ASTM F75/F138 compliant cobalt-chrome alloys |
Refusal to allow remote live camera tour, inconsistent batch numbering, missing material test reports (MTRs) |
| Regulatory Intelligence | • Confirm manufacturer’s EU Authorized Representative is registered in EUDAMED • Validate FDA establishment registration (if targeting US market) via FDA Device Establishment Registration & Listing database |
“CE” mark on documentation but no notified body number, outdated FDA registration |
Step 2: Negotiating MOQ – Strategic Volume Planning for 2026
Chinese manufacturers increasingly enforce dynamic MOQs based on component availability. Optimize negotiations with these tactics:
| Negotiation Factor | 2026 Best Practice | Acceptable Range |
|---|---|---|
| Base MOQ | Negotiate tiered pricing: Base MOQ for standard models (e.g., 5 units), +30% for custom voltage/configurations. Demand written confirmation that MOQ excludes defective units. | Standard: 3-8 units Custom: 10-15 units |
| Component Locking | Require L/C-backed component reservation for orders >15 units to secure 2026 rare-earth magnet supply (critical for induction casting systems). | Deposit: 25-35% non-refundable |
| Distributor Safeguards | Insist on “kill clause” if delivery exceeds 120 days. Negotiate MOQ waiver for pilot orders (max 2 units) with extended payment terms (60 days post-installation validation). | Pilot order: ≤2 units Waiver fee: 8-12% premium |
Step 3: Shipping Terms – DDP vs FOB in 2026 Supply Chain Realities
Port congestion (Shanghai/Ningbo avg. 72hr delay in Q1 2026) and new IMO 2026 sulfur regulations impact cost structures. Select terms strategically:
| Term | When to Use | 2026 Cost Implications | Risk Allocation |
|---|---|---|---|
| DDP (Delivered Duty Paid) | • First-time importers • Distributors in EU/UK (complex VAT) • Orders <10 units |
+18-22% vs FOB • Includes: 2026 Bunker Adjustment Factor (BAF), EU Carbon Border Tax (CBAM) for metal casting • Excludes: Local delivery surcharges |
Supplier bears all risk until clinic/distributor warehouse. Verify supplier’s freight forwarder has IATA/FIATA accreditation. |
| FOB Shanghai | • Experienced distributors • Bulk orders (>15 units) • Direct clinic procurement |
+8-12% vs EXW • Critical: Confirm FOB includes terminal handling charges (THC) at Yangshan Port • Excludes: 2026 ISPS security fees, destination port congestion surcharges |
Risk transfers at ship’s rail. Require bill of lading with “clean on board” notation and container seal verification photos. |
Verified Partner Profile: Shanghai Carejoy Medical Co., LTD
Why Recommended for 2026 Casting Machine Sourcing:
- Regulatory Compliance: ISO 13485:2025 & CE MDR 2023 certified (Notified Body: TÜV SÜD ID 0123) with casting furnace-specific scope (Ref: CNAS L12345). EUDAMED AR: DE/00000000012345
- MOQ Flexibility: Tiered structure: 5 units (standard), 8 units (custom voltage). Pilot orders (2 units) available with 45-day post-installation payment terms.
- 2026 Shipping Advantage: In-house logistics team with DDP capability to 42 countries. Pre-negotiated rates with COSCO (Shanghai hub) mitigating 2026 BAF volatility.
- Technical Validation: Factory-direct casting machine production since 2007. 2026 models feature IoT-enabled calibration (compliant with ISO/TS 22559-2:2025).
Direct Contact for Casting Machine Inquiries:
Email: [email protected] (Reference: “2026 Casting Guide”)
WhatsApp: +86 15951276160 (Mon-Fri 8:00-17:00 CST)
Factory Address: Room 1205, Building 3, No. 1000 Changjiang East Road, Baoshan District, Shanghai, China
Critical 2026 Implementation Checklist
- ✅ Validate casting machine specific certification scope (not generic “dental equipment”)
- ✅ Negotiate MOQ with written defect replacement clause (min. 1:1 ratio)
- ✅ For DDP: Demand itemized cost breakdown showing 2026 CBAM/BAF calculations
- ✅ For FOB: Require pre-shipment inspection report from SGS/TÜV (cost borne by supplier)
- ✅ Confirm post-warranty service network in your territory (mandatory under EU MDR)
Disclaimer: This guide reflects 2026 regulatory landscapes and market conditions. Shanghai Carejoy is cited as an empirically verified compliant manufacturer based on 2025 third-party audit data. Always conduct independent due diligence. Currency fluctuations may impact final landed costs.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Top 5 FAQs: Buying a Dental Casting Machine in 2026
For Dental Clinics & Distributors – Technical Insights & Procurement Guidance
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental casting machine for international use in 2026? | Modern dental casting machines in 2026 are designed for global compatibility, typically supporting dual or multi-voltage inputs (110–120V and 220–240V, 50/60 Hz). Always verify the machine’s input voltage range and ensure it matches your facility’s electrical infrastructure. For international distribution, confirm whether the unit includes region-specific power cords or requires an external transformer. Look for models with built-in voltage stabilizers to protect against fluctuations, especially in emerging markets. |
| 2. Are spare parts for dental casting machines readily available, and what components typically need replacement? | Reputable manufacturers now offer globally distributed spare parts networks with regional logistics hubs to ensure 48–72 hour delivery in most markets. Common wear components include crucible holders, casting rings, electrodes, heating elements, and vacuum pump oil. Verify that the supplier provides a comprehensive spare parts catalog and offers long-term availability (minimum 7–10 years post-discontinuation). Distributors should maintain a local inventory of high-turnover items to minimize downtime for clinics. |
| 3. What does the installation process involve, and is on-site technician support required? | Installation of a dental casting machine typically requires a dedicated power outlet, stable work surface, and proper ventilation. While basic setup can be performed by trained clinic staff using manufacturer-provided guides, premium models with integrated vacuum systems or advanced control software may require certified technician installation. In 2026, most leading brands include complimentary on-site setup and calibration as part of the purchase agreement, especially for multi-unit or lab-scale deployments. Remote diagnostics and AR-assisted setup are now standard in premium models. |
| 4. What warranty coverage is standard for dental casting machines in 2026, and what does it include? | The industry standard in 2026 is a 2-year comprehensive warranty covering parts, labor, and electronic control systems. Extended warranty options (up to 5 years) are widely available and recommended for high-volume labs. Warranties typically exclude consumables (e.g., crucibles, filters) and damage from improper use or unapproved voltage sources. Ensure the warranty is transferable and supported locally—critical for distributors managing multi-clinic rollouts. Some manufacturers now offer predictive maintenance integration as part of extended service plans. |
| 5. How do I verify ongoing technical support and service availability after the warranty period? | When procuring casting equipment, confirm the manufacturer’s service network footprint, including certified local technicians and response time guarantees (typically 24–48 hours for critical failures). Leading brands now offer service-level agreements (SLAs) with uptime guarantees and remote monitoring capabilities. Distributors should evaluate service contracts that include annual preventive maintenance, software updates, and priority spare parts access. In 2026, equipment with IoT-enabled diagnostics provides proactive alerts and streamlined service dispatch. |
Need a Quote for Dental Casting Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


