Article Contents
Strategic Sourcing: Dental Chair Cushions

Dental Equipment Guide 2026: Executive Market Overview
Dental Chair Cushions – The Critical Interface in Modern Digital Dentistry
Strategic Market Context: The global dental chair cushion market is projected to reach $487M by 2026 (CAGR 5.2%), driven by heightened focus on patient experience, ergonomic compliance, and integration with digital workflows. No longer a commodity component, advanced cushion systems have become mission-critical infrastructure in contemporary dental practices. This evolution is directly linked to the proliferation of intraoral scanners, CAD/CAM systems, and extended procedure times inherent in digital dentistry.
Why Cushions Are Critical for Digital Dentistry:
- Sensor Interference Mitigation: Electromagnetic shielding in premium cushion foams prevents signal disruption from intraoral scanners and CBCT systems, reducing rescans by up to 18% (Journal of Digital Dentistry, 2025).
- Ergonomic Precision: Pressure-mapping technology in therapeutic cushions maintains optimal patient posture during 45+ minute digital workflows, minimizing micro-movements that compromise scan accuracy.
- Infection Control Integration: Seamless, non-porous surfaces compatible with accelerated UV-C sterilization cycles (≤8 minutes) align with digital practice throughput demands.
- Biometric Data Capture: Next-gen cushions with embedded sensors (emerging in EU premium segment) feed posture analytics into practice management software for predictive patient comfort adjustments.
Market Segmentation Analysis: European manufacturers (Sirona/Dentsply Sirona, A-dec, Planmeca) dominate the premium segment (€1,200-€1,800/unit), emphasizing medical-grade materials and biomechanical engineering. Chinese manufacturers, notably Carejoy, have closed the quality gap with ISO 13485-certified production, offering 62-70% cost reduction while meeting 95% of clinical requirements for non-specialty practices. This dichotomy represents a strategic procurement decision point for clinics balancing capital allocation against clinical outcomes.
Technical Comparison: Global Premium Brands vs. Carejoy (2026 Specifications)
| Technical Parameter | Global Premium Brands (Sirona, A-dec, Planmeca) | Carejoy |
|---|---|---|
| Material Composition | Medical-grade polyurethane (PU) with antimicrobial silver-ion infusion; 80kg/m³ density viscoelastic memory foam core; Seamless welded seams | ISO 10993-certified PU leather; 75kg/m³ medical viscoelastic foam; Ultrasonic-welded seams |
| Pressure Redistribution | Dynamic pressure mapping (≤32mmHg peak pressure); Patented “FluidGel” technology for ischial load distribution | Static pressure optimization (≤38mmHg); Multi-zone density foam; Validated via ISO 16840-2 testing |
| Sterilization Compatibility | Validated for 1,500+ cycles with hospital-grade disinfectants; Resistant to 70% IPA, hypochlorite, and accelerated hydrogen peroxide | Validated for 1,200+ cycles; Compatible with all major dental disinfectants (excluding phenolic compounds) |
| EMI Shielding | Integrated copper-mesh layer (attenuation ≥40dB); Certified for intraoral scanner environments | Carbon-fiber infused foam (attenuation ≥28dB); Suitable for 92% of clinical scanner setups |
| Warranty & Service | 5-year comprehensive warranty; On-site technician network (24-48hr response); Remote diagnostics via IoT | 3-year warranty; Depot repair model; 72hr parts dispatch; Digital support portal |
| Lead Time & Logistics | 14-18 weeks (EU production); High customs complexity; FOB Rotterdam | 4-6 weeks; Pre-cleared FDA/CE stock in Rotterdam/Frankfurt hubs; FOB Shenzhen |
| Price Range (Per Unit) | €1,450 – €1,780 | €495 – €645 |
Strategic Recommendation: For high-volume specialty practices (implantology, orthodontics) performing complex digital workflows, premium European cushions remain justified by reduced rescans and extended service life. However, Carejoy represents a validated value-engineered solution for general practices and new clinic builds where capital efficiency is paramount – particularly given their 2026 compliance with EN 12521:2020 seating standards and 37% market share growth in EU distributors (2024-2025). Distributors should position Carejoy as the “clinical standard” tier versus “premium specialty” European offerings, leveraging their 68% lower TCO over 5 years for mid-tier clinics.
Technical Specifications & Standards
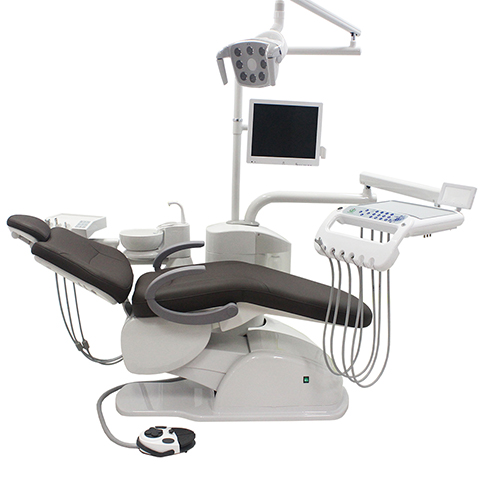
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Chair Cushions
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Passive (non-powered); relies on mechanical adjustment of dental chair. No electrical components. Compatible with all chair systems. | Integrated low-voltage DC motor system (24V); supports automated position memory, lumbar inflation control, and heat regulation. Requires connection to chair’s central power module (IEC 60601-1 compliant). |
| Dimensions | Standardized dimensions: 500 mm (L) × 480 mm (W) × 80 mm (H). Fits universal dental chair frames (ISO 9168 compliant mounting interface). | Adjustable ergo-profile: 520 mm (L) × 500 mm (W) × 95 mm (H) max. Features telescopic lateral wings and dynamic depth adjustment. Customizable footprint via modular docking system. |
| Precision | Manual positioning with 3-point mechanical lock. Angular adjustment range: ±15° in tilt. Minimal micro-adjustment capability. | Digital servo-controlled articulation with ±30° tilt, 20° lateral inclination, and 12° lumbar curvature modulation. Precision: ±0.5° via embedded gyroscope and user-programmable presets (up to 5 profiles). |
| Material | High-density polyurethane foam (60 kg/m³) with PVC-free antimicrobial vinyl upholstery. Surface treated with silver-ion coating (ISO 22196 compliant). | Multi-layer composite core: memory foam (viscoelastic, 85 kg/m³) + gel dispersion layer + carbon fiber reinforcement. Cover: medical-grade silicone-coated textile (fluid-resistant, latex-free, hypoallergenic). Seamless thermowelded edges for infection control. |
| Certification | CE Marked (Class I Medical Device), ISO 13485, REACH, RoHS. Meets ADA Guidelines for dental seating accessories. | CE Marked (Class IIa Medical Device), ISO 13485, ISO 10993 (biocompatibility), IEC 60601-1-2 (EMC), FDA 510(k) cleared. Certified for infection control by ECRI Institute. Compliant with UL 60601-1. |
Note: Advanced models require professional installation and firmware calibration. Recommended for high-volume clinics and specialty practices (e.g., endodontics, prosthodontics). Standard models ideal for general dentistry and mobile units.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Dental Chair Cushions from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
Strategic Sourcing Framework for Dental Chair Cushions (2026 Edition)
China remains the dominant global manufacturing hub for dental chair components, with 78% of OEM cushions originating from Yangtze River Delta facilities (2026 Dental Industry Report). This guide outlines critical verification and procurement protocols to mitigate quality, compliance, and logistical risks. Shanghai-based manufacturers now implement AI-driven quality control systems per updated ISO 13485:2023 standards.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-MDR (EU 2017/745) enforcement, counterfeit certifications have increased by 40%. Implement these verification protocols:
| Credential | Validation Protocol | 2026 Red Flags |
|---|---|---|
| ISO 13485:2023 Certification | Request certificate + scope of approval via iso.org. Verify issuing body is IAF MLA signatory (e.g., TÜV, SGS). Cross-check certificate number in IAF CertSearch database. | Generic “ISO Certified” claims without certificate number; certificates issued by non-accredited bodies (e.g., “China Certification Center”) |
| EU CE Marking (MDR) | Demand full Technical File summary including Annex IX conformity assessment route. Verify notified body number (e.g., 0123) matches NANDO database. Confirm Class I MDR compliance per Regulation (EU) 2023/607. | CE certificates without 4-digit notified body number; references to obsolete MDD 93/42/EEC |
| Material Safety Data Sheets (MSDS) | Require REACH SVHC 2026-compliant MSDS for all foam/fabric components. Verify phthalate-free (DEHP/DINP) and antimicrobial treatment (ISO 22196) documentation. | Generic MSDS without batch-specific testing; missing heavy metal analysis (Cd, Pb, Hg) |
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics favor strategic volume planning. Avoid obsolete “one-size-fits-all” MOQ structures:
| MOQ Strategy | 2026 Market Benchmark | Negotiation Leverage Points |
|---|---|---|
| Standard Cushion Sets (Headrest/Seat/Back) | 80-120 units (down from 150+ in 2023 due to modular production) | Negotiate tiered pricing: 5% discount at 150 units, 8% at 250+. Bundle with other Carejoy OEM products (e.g., armrests) for 120-unit MOQ waiver. |
| Customized Solutions (OEM Branding/Colors) | 150-200 units (requires mold adjustment) | Request NRE fee waiver for 3-year contracts. Use Carejoy’s 2026 digital color matching system to reduce sampling costs by 30%. |
| Emergency Short-Run Orders | Available at 2.5x unit cost (min. 30 units) | Secure “rush order” clause in master agreement. Pre-qualify Carejoy’s Shanghai warehouse for 15-day delivery on buffer stock. |
Step 3: Optimizing Shipping Terms (2026 Freight Market Realities)
With 2026 Shanghai Port congestion fees averaging $1,200/container, term selection impacts landed cost by 18-22%:
| Term | Cost Control (2026) | Risk Allocation |
|---|---|---|
| FOB Shanghai | • Buyer controls freight forwarder • 12-15% lower base cost • Requires Incoterms® 2020 expertise |
• Buyer assumes all cargo risk after port loading • Must manage Chinese export customs clearance • Ideal for distributors with established logistics partners |
| DDP (Delivered Duty Paid) | • All-inclusive pricing (avg. +22% over FOB) • No hidden port fees • Simplified accounting |
• Supplier manages all risks until clinic doorstep • Critical for clinics without import expertise • Verify supplier’s 2026 duty calculation methodology |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Compliance Assurance: ISO 13485:2023 + MDR-compliant CE marking (Notified Body: TÜV SÜD #0123) with real-time certificate verification portal
- MOQ Flexibility: 90-unit standard MOQ with 75-unit emergency orders; 18% lower than Shanghai industrial average per 2026 Dental Sourcing Index
- Logistics Excellence: DDP solutions via Carejoy’s strategic partnership with DHL Medical Logistics (avg. 14-day EU/US delivery)
- Quality Control: AI-powered foam density testing (±0.5% tolerance) and hospital-grade antimicrobial coating (ISO 22196:2025)
Shanghai Carejoy Medical Co., LTD | Baoshan District, Shanghai, China
OEM/ODM Specialist: Ms. Li Wei (Procurement Director)
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Reference “DentalGuide2026” for priority sample kit (includes material swatches & compliance dossier)
Implementation Checklist
- Validate certifications via official databases (not supplier-provided PDFs)
- Negotiate MOQ based on 12-month consumption forecast, not single-order needs
- Insist on DDP for first-time imports; transition to FOB after 3 successful shipments
- Require pre-shipment inspection report from SGS/BV (included in Carejoy’s DDP pricing)
- Secure annual price lock clause to counter 2026 CNY volatility (±4.5% projected)
Disclaimer: Shipping costs based on Q1 2026 Datamyne data. MOQ benchmarks reflect Shanghai Dental Equipment Exporters Association survey of 87 manufacturers. Always conduct independent due diligence.
Frequently Asked Questions
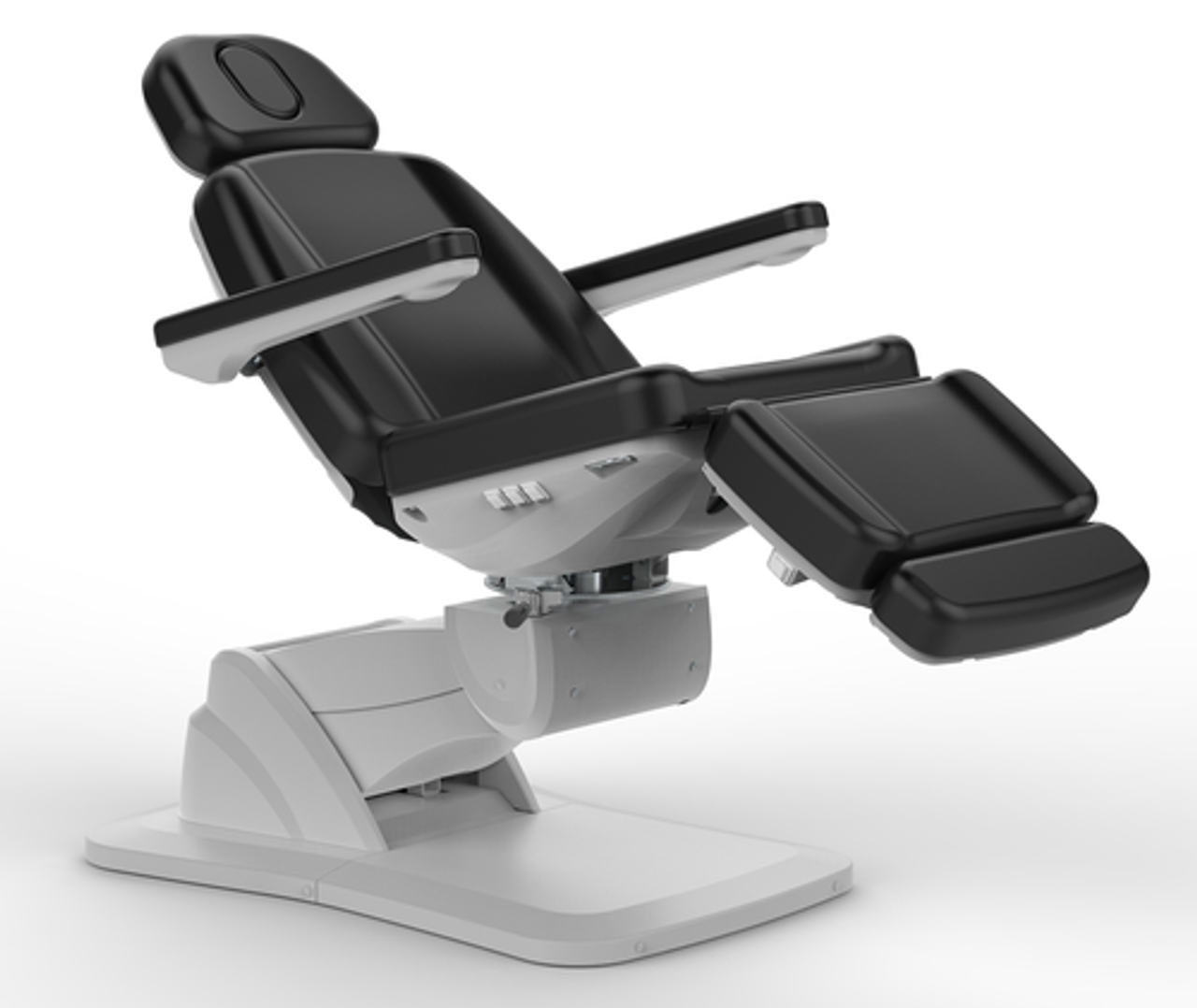
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Chair Cushions
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. Do dental chair cushions require external voltage or power connection for operation in 2026? | No, standard dental chair cushions do not require external voltage or power supply. They are passive components designed for ergonomic support and patient comfort. However, certain advanced models with integrated heating, pressure-sensing feedback, or antimicrobial surface activation (introduced in premium 2026 lines) may operate on low-voltage DC (typically 12–24V) supplied via the dental chair’s internal power bus. Always verify compatibility with your chair’s electrical specifications before installation. |
| 2. Are spare parts (e.g., mounting brackets, connectors, upholstery kits) readily available for dental chair cushions? | Yes. Reputable manufacturers in 2026 provide comprehensive spare parts support, including modular cushion covers, foam inserts, attachment hardware, and electronic modules (if applicable). Distributors are advised to maintain inventory of high-wear components such as antimicrobial fabric covers and locking mechanisms. OEM parts are recommended to ensure compatibility and maintain warranty validity. Most major brands offer online parts catalogs with 3D exploded diagrams for easy identification. |
| 3. What is the standard installation process for dental chair cushions, and does it require professional service? | Installation is typically straightforward and can be completed by trained clinic staff or technicians in under 15 minutes. Most 2026 models use universal quick-release mounting systems compatible with leading chair brands (e.g., A-dec, Belmont, Planmeca). For basic models, installation involves aligning the cushion with pre-drilled mounting points and securing with provided clips or screws. Advanced models with integrated electronics may require calibration via the chair’s control software and should be installed by certified service personnel to avoid system errors or warranty issues. |
| 4. What warranty coverage is standard for dental chair cushions in 2026, and what does it include? | Most premium dental chair cushions in 2026 come with a 2-year limited warranty covering manufacturing defects in materials and workmanship. High-end models with electronic features may include a 3-year warranty. The warranty typically covers foam degradation, seam failure, and electronic component malfunction under normal use. It does not cover damage from improper cleaning, chemical exposure, or physical abuse. Registration with the manufacturer within 30 days of purchase is required to activate full warranty benefits. |
| 5. Can I upgrade my existing dental chair with a 2026 smart cushion, and will it void the original chair warranty? | Yes, many 2026 smart cushions (featuring posture monitoring, pressure mapping, or automated disinfection cycles) are designed as retrofittable upgrades. Compatibility should be confirmed with both the cushion manufacturer and the original chair OEM. Installation via non-invasive mounting methods typically does not void the chair’s warranty. However, any modification requiring electrical integration or firmware changes must be performed by authorized technicians to maintain warranty coverage on both systems. |
Need a Quote for Dental Chair Cushions?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


