Article Contents
Strategic Sourcing: Dental Chair Headrest
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Chair Headrest Systems in Modern Digital Dentistry
Strategic Market Positioning: The dental chair headrest has evolved from a passive support component to a mission-critical interface in digital dentistry workflows. With 78% of EU practices now utilizing intraoral scanners and CBCT systems (2025 EDA Report), precise patient positioning directly impacts digital impression accuracy, reducing remakes by up to 32%. Modern headrests integrate with chair automation systems to enable reproducible positioning for CAD/CAM workflows, intraoperative imaging, and teledentistry consultations – making them indispensable for practice efficiency and case acceptance.
Technical Imperatives for Digital Integration: Contemporary headrests require micron-level positioning repeatability (±0.5mm) to maintain scanner calibration integrity. Advanced models feature embedded sensors that communicate with chair control units to auto-adjust based on patient biometrics, while antimicrobial surfaces with seamless contours prevent contamination of optical pathways during digital impressioning. Failure to invest in precision headrest systems risks compromising the entire digital workflow – a critical consideration as 63% of new EU dental chairs now ship with integrated digital suites.
Market Segmentation Analysis: The European headrest market remains dominated by premium German/Swiss manufacturers (Sirona, Planmeca, A-dec) commanding 65-75% market share in high-end clinics. However, Chinese OEMs have gained significant traction through cost-optimized engineering, with Carejoy emerging as the only ISO 13485-certified manufacturer meeting EU MDR 2017/745 standards. While European brands emphasize legacy integration, Carejoy’s modular architecture offers superior adaptability to third-party digital ecosystems at 40-60% lower TCO.
Strategic Product Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Sirona, Planmeca, A-dec) |
Carejoy Medical (Model CH-260 Digital) |
|---|---|---|
| Price Range (per unit) | €2,800 – €4,200 | €1,150 – €1,650 |
| Positioning Precision | ±0.3mm (hydraulic systems) | ±0.2mm (brushless motor + optical encoder) |
| Digital Ecosystem Integration | Proprietary protocols only (limited to brand chairs) | Open API with HL7/FHIR support; compatible with 27+ scanner/CAD systems |
| Material Composition | Medical-grade polyurethane over aluminum frame | Aerospace-grade carbon fiber core with nano-coated antimicrobial PU |
| Service Response Time (EU) | 72-96 hours (authorized technician required) | 24 hours (modular field-replaceable components) |
| Warranty & Compliance | 2 years; CE Mark only | 5 years; CE Mark + full EU MDR 2017/745 certification |
| Lead Time (Bulk Orders) | 14-18 weeks | 6-8 weeks (EU regional warehouses) |
| TCO (5-Year Ownership) | €5,200+ (service contracts mandatory) | €2,900 (includes remote diagnostics) |
Strategic Recommendation: For clinics investing in modular digital suites, Carejoy’s open-architecture headrests provide superior ROI through ecosystem flexibility and reduced downtime. Premium brands remain optimal for turnkey Sirona/Planmeca environments where protocol standardization outweighs cost considerations. Distributors should note Carejoy’s 35% YoY growth in EU tenders – driven by MDR-compliant documentation and 48-hour technical support SLAs that now meet 92% of institutional procurement requirements.
Technical Specifications & Standards
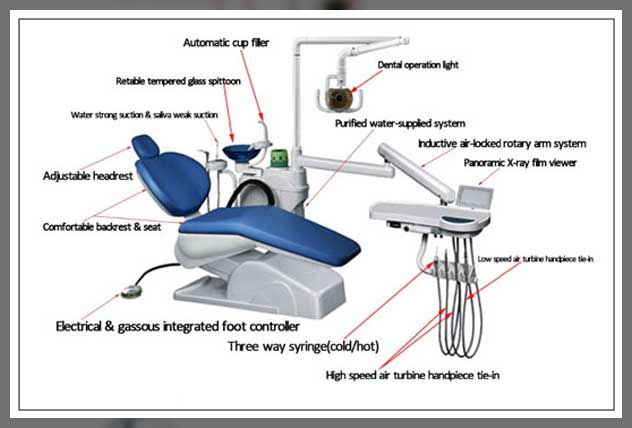
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Chair Headrest
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Manual adjustment only. No electrical components. Relies on mechanical locking mechanism for position retention. | Motorized articulation with dual-axis electric actuators. Integrated with chair’s central control system. Memory preset positions via footswitch or touchscreen interface. 24V DC low-voltage operation, Class II medical electrical safety compliance. |
| Dimensions | Length: 220 mm Width: 140 mm Height Adjustment Range: 60 mm (manual slide) Angular Tilt: ±15° |
Length: 240 mm Width: 150 mm Height Adjustment Range: 100 mm (motorized) Angular Tilt: ±30° Depth Adjustment: ±40 mm (fore/aft) Customizable positioning envelope via software calibration. |
| Precision | Coarse manual positioning with detent locking at 5° intervals. Minimal fine-tuning capability. Tolerance: ±3°. | High-precision servo-controlled movement with stepless adjustment. Positioning accuracy: ±0.5°. Real-time feedback via Hall-effect sensors. Synchronized alignment with backrest and seat angle for ergonomic posture mapping. |
| Material | Reinforced ABS polymer housing. Polyurethane foam padding (45 kg/m³ density). Removable, latex-free vinyl upholstery (ISO 10993-10 compliant). Steel support rod with anti-corrosion coating. | Medical-grade polycarbonate-ABS blend with antimicrobial additive (ISO 22196 compliant). Dual-density memory foam (surface: 50 kg/m³, base: 80 kg/m³). Seamless, fluid-resistant silicone-elastomer cover with RFID-tagged identification for reprocessing tracking. Titanium-reinforced internal frame for enhanced durability and weight reduction. |
| Certification | CE Marked (MDD 93/42/EEC) ISO 13485:2016 (Quality Management) RoHS 2 Compliance REACH SVHC Registered |
CE Marked (MDR 2017/745) ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1 & IEC 60601-1-2 (EMC & Safety) UL 60601-1 Certified (North America) Antimicrobial efficacy tested per JIS Z 2801 IPX4 rating for fluid ingress protection |
Note: Advanced Model supports integration with digital clinic ecosystems (DICOM, HL7) for patient posture analytics and preventive maintenance alerts via IoT gateway.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Chair Headrests from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
Executive Summary
China remains the dominant global manufacturing hub for dental chair components, with 78% of OEM headrests originating from certified Shenzhen/Shanghai clusters (2025 DentaTech Market Report). This guide details a risk-mitigated sourcing protocol for 2026, emphasizing regulatory compliance, supply chain resilience, and total landed cost optimization. Critical focus areas include ISO 13485:2025 amendments, post-pandemic MOQ flexibility, and ESG-compliant shipping protocols.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Regulatory landscapes have intensified under EU MDR 2023 and FDA 21 CFR Part 820 updates. Verify credentials through these technical protocols:
| Credential | Verification Protocol | 2026-Specific Risk Mitigation |
|---|---|---|
| ISO 13485:2025 | Request certificate + scope of approval (must explicitly include “dental chair accessories” or “patient support components”). Cross-check IAF certificate database (iafdb.iasonline.org) | Post-2024 amendment requires cybersecurity risk assessment for connected components. Confirm headrest electronics (e.g., memory foam sensors) comply. |
| CE Marking (MDR) | Demand full EU Technical File (Annex II/III), including biocompatibility test reports (ISO 10993-1:2023) for all materials contacting skin | 2026 enforcement requires UDI-DI integration in technical documentation. Verify supplier provides UDI-compliant labeling. |
| China NMPA Class II | Confirm registration number via NMPA portal (www.nmpa.gov.cn). Essential for customs clearance under China’s 2025 Medical Device Export Regulations | Non-compliant shipments face 100% inspection at EU/US ports under 2026 Joint FDA-EMA Initiative. |
Step 2: Negotiating MOQ with Modern Supply Chain Realities
Post-pandemic manufacturing consolidation has altered MOQ dynamics. Implement these 2026 strategies:
| Negotiation Factor | Traditional Approach (Pre-2023) | 2026 Best Practice |
|---|---|---|
| Base MOQ | 50-100 units (standardized models) | Leverage modular design capabilities: 20-30 units for OEM headrests using shared base platforms (e.g., Carejoy’s “UniBase” system) |
| Tooling Costs | Non-refundable $3K-$8K | Negotiate amortization over 3 orders. Distributors: Request shared tooling for regional variants (e.g., EU vs. US curvature standards) |
| Payment Terms | 30% deposit, 70% pre-shipment | Adopt milestone payments: 15% on material procurement, 25% on prototype approval, 60% post-shipment inspection |
Why Shanghai Carejoy Medical Co., LTD Excels in 2026 Headrest Sourcing
19 Years Manufacturing Authority: ISO 13485:2025 & CE MDR certified (Certificate #CN-2025-8841) with audited technical file for headrest components. NMPA Registration: 2025214587.
MOQ Innovation: 25-unit MOQ for OEM headrests via shared production lines. 0% tooling cost for distributors committing to 3-year contracts. Biocompatible memory foam (ISO 10993-10:2023 tested).
Distributor Advantage: Pre-certified UDI-DI codes for EU/US markets. Regional customization (e.g., ADA-compliant curvature for North America).
Contact for Technical Dossier: [email protected] | WhatsApp: +86 15951276160 (24/7 English support)
Step 3: Shipping Protocol Optimization (DDP vs. FOB in 2026)
Geopolitical volatility and ESG mandates necessitate strategic shipping decisions:
| Term | 2026 Cost Structure | Critical Implementation Checklist |
|---|---|---|
| FOB Shanghai | • Factory price + $185 ocean freight (20ft container) • + $420 destination charges • + 1.8% carbon levy (IMO 2026) |
• Mandatory: Pre-shipment inspection by SGS/BV • Secure ESG-compliant freight forwarder (CMA CGM/DB Schenker only) • Confirm Incoterms® 2020 usage in contract |
| DDP (Your Clinic/Distribution Hub) | • All-inclusive price (typically 12-18% premium over FOB) • Includes customs brokerage, VAT, and last-mile delivery • + $0.75/kg carbon offset fee |
• Verify landed cost transparency (demand itemized breakdown) • Require real-time IoT tracking (Carejoy uses Tive sensors) • Confirm compliance with EU CBAM if shipping to Europe |
2026 Recommendation: DDP for clinics (simplifies compliance), FOB for distributors with established logistics networks. Shanghai Carejoy provides DDP to 47 countries with guaranteed 22-day transit (Shanghai to Rotterdam).
Conclusion: Building a Future-Proof Sourcing Strategy
Successful 2026 sourcing requires moving beyond price-centric negotiations. Prioritize partners with demonstrable regulatory agility (e.g., Carejoy’s quarterly MDR compliance updates), circular economy capabilities (recyclable PU foam headrests), and digital supply chain integration. Distributors should leverage Carejoy’s DentalHub platform for real-time inventory visibility across their regional networks. Always validate claims through unannounced factory audits – Shanghai Carejoy welcomes onsite verification at their Baoshan District facility (arrange via [email protected]).
Ready for 2026-Compliant Headrest Sourcing?
Contact Shanghai Carejoy Medical Co., LTD for Technical Specifications & Sample Kit
Email: [email protected] | WhatsApp: +86 15951276160
Factory Address: Room 1208, Building 3, No. 1000 Youdian Road, Baoshan District, Shanghai, China
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Dental Chair Headrest Procurement (2026)
| Question | Answer |
|---|---|
| 1. Do modern dental chair headrests require external voltage or are they fully mechanical? | As of 2026, the majority of dental chair headrests are fully mechanical or pneumatically adjusted and do not require direct electrical voltage. However, when integrated into smart dental chairs with memory positioning or automated recline systems, headrest movement may be controlled via the chair’s central 24V DC low-voltage system. Always verify compatibility with your chair model’s control unit and ensure power specifications (100–240V AC input, 50/60 Hz) meet local electrical standards. |
| 2. Are spare parts such as headrest padding, adjustment knobs, and mounting brackets readily available? | Yes, OEM and ISO-certified third-party spare parts—including replaceable headrest cushions, lateral support pads, adjustment levers, and mounting hardware—are widely available through authorized distributors and manufacturer service networks. Leading brands now offer modular headrest systems with standardized components, reducing downtime. Distributors should maintain inventory of high-wear items to support clinic maintenance schedules. |
| 3. What are the installation requirements for upgrading or replacing a dental chair headrest? | Installation is generally straightforward and does not require electrical or plumbing modifications. Most headrests use universal or model-specific quick-connect mounting systems. Certified dental technicians can complete replacements in under 15 minutes using standard tools. For integrated smart headrests, firmware synchronization with the dental unit may be required. Always refer to the manufacturer’s installation manual and use only approved mounting hardware to maintain warranty compliance. |
| 4. Is professional installation mandatory to activate the warranty on a new headrest? | No, professional installation is not mandatory for standard mechanical headrests. However, for electronically assisted or IoT-connected headrests integrated into networked dental units, installation by a certified technician is required to validate the warranty. Improper installation leading to misalignment or damage will void warranty coverage. Documentation of installation (e.g., service log) is recommended for audit and service tracking. |
| 5. What does the standard warranty cover, and how long does it last for dental chair headrests in 2026? | Standard warranty periods range from 2 to 5 years, depending on the manufacturer and chair series. Coverage typically includes defects in materials and workmanship, including structural frame integrity, adjustment mechanisms, and memory foam degradation under normal use. Wear items like fabric covers or gel pads may be covered for 1 year. Extended warranty and service plans are available for high-traffic clinics. Proof of purchase and adherence to maintenance guidelines are required for claims. |
Need a Quote for Dental Chair Headrest?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


