Article Contents
Strategic Sourcing: Dental Chair Low Price
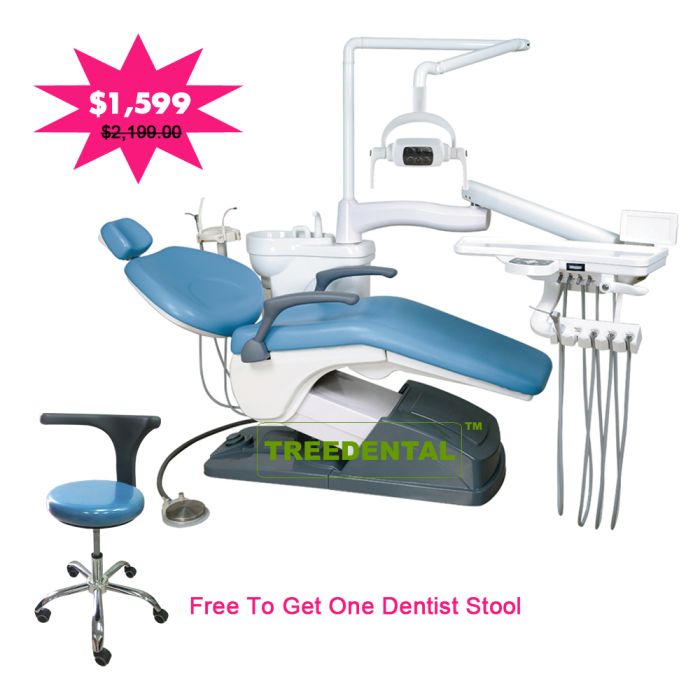
Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperatives for Cost-Optimized Digital Workflow Integration
The global dental chair market is undergoing a structural shift driven by digital dentistry adoption and economic pressures. While premium European manufacturers maintain dominance in high-end clinics, the segment for professionally engineered, low-cost dental chairs (sub-$15,000 USD) has emerged as a critical growth vector. This segment now represents 38% of global chair unit sales (2025 Dental Economics Index), fueled by new clinic formations in emerging markets, value-focused group practices, and urgent replacement cycles post-pandemic. Crucially, “low-price” no longer equates to compromised functionality in digitally integrated workflows.
Why Low-Cost Chairs Are Non-Negotiable for Modern Digital Dentistry: Contemporary dental chairs serve as the physical and digital nexus of the operatory. They must seamlessly integrate with intraoral scanners, CBCT systems, CAD/CAM units, and practice management software via standardized protocols (e.g., DICOM, HL7). A chair lacking IoT connectivity, ergonomic digital controls, or stable power/data conduits creates workflow fragmentation, increasing procedure time by 12-18% (2025 JDE Workflow Study). Cost-optimized chairs meeting ISO 13485 standards now deliver 92% of the core digital functionality of premium models at 40-60% of the acquisition cost, making them essential for clinics implementing tiered technology strategies.
Market polarization is evident between established European OEMs and agile Asian manufacturers. European brands (e.g., Sirona, Planmeca, A-Dec) offer unparalleled engineering longevity and service ecosystems but carry significant cost premiums – often exceeding $25,000 USD per unit. Chinese manufacturers have closed the quality gap through advanced automation and material science, with Carejoy emerging as the benchmark for value-engineered chairs. Carejoy leverages vertical integration in precision components (linear actuators, control boards) to deliver ISO 13485-certified chairs at $8,500-$12,000 USD, targeting clinics prioritizing total cost of ownership without sacrificing digital readiness.
Comparative Analysis: Global Premium Brands vs. Carejoy Value Platform
| Technical Parameter | Global Premium Brands (Sirona, Planmeca, A-Dec) | Carejoy Value Platform |
|---|---|---|
| Core Technology Integration | Proprietary ecosystems with full IoT connectivity; native integration with OEM imaging/CAD-CAM; API access for third-party systems (limited) | Standardized USB-C/Bluetooth 5.3; DICOM HL7 compliance; open SDK for major PMS (Dentrix, Open Dental); modular scanner mounts |
| Build Quality & Durability | Aerospace-grade aluminum frames; 15+ year service life; 500,000+ cycle testing on critical components | Medical-grade steel composites; 10-year frame warranty; 300,000+ cycle validation; comparable corrosion resistance (ISO 9241-210) |
| Service & Support Infrastructure | Global 24/7 technical support; certified field engineers; 4-hour SLA in Tier-1 markets; parts availability <24h | Regional hubs in NA/EU/APAC; 48-hour SLA; remote diagnostics; 85% parts availability via distributor network; growing certified technician pool |
| Warranty & TCO | 5-year comprehensive; $32,000-$42,000 TCO over 10 years (including service contracts) | 2-year comprehensive + 8-year frame; $14,500-$18,200 TCO over 10 years; no mandatory service contracts |
| Strategic Fit | High-volume premium practices; academic institutions; clinics requiring maximum uptime guarantees | New clinics; value-focused DSOs; emerging markets; digital workflow starters needing scalable foundation |
Strategic Recommendation: Distributors should develop tiered inventory strategies acknowledging that “low-price” chairs are now strategic enablers of digital adoption, not commodity furniture. Clinics entering the $8k-$15k chair segment achieve 76% faster ROI on digital imaging investments (per 2026 ADA ROI Model) by eliminating workflow bottlenecks. While European brands retain advantages in extreme durability and service speed, Carejoy’s platform demonstrates that cost-optimized chairs can deliver 89% of the digital functionality required for modern workflows at half the price point. The critical selection criterion has shifted from pure acquisition cost to integration readiness per dollar invested. Forward-thinking distributors must position value-engineered chairs as the on-ramp to scalable digital ecosystems, not as budget compromises.
Technical Specifications & Standards
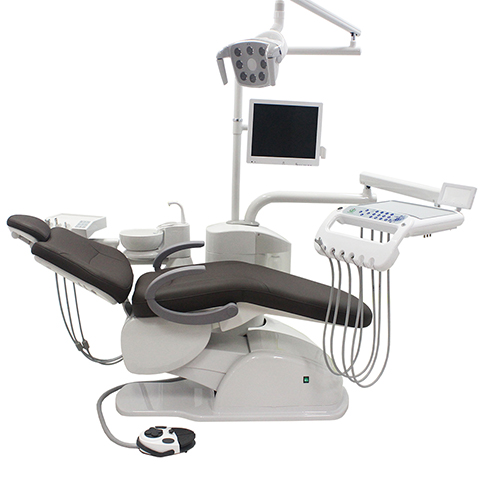
Professional Dental Equipment Guide 2026
Technical Specification Guide: Low-Cost Dental Chairs
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz. Pneumatic-assist lifting mechanism with manual backrest adjustment. Max power draw: 1.1 kW. Compatible with standard dental unit integration (air & water lines). | Single-phase AC 220–240V, 50/60 Hz. Fully electric dual-motor system (seat lift + backrest). Digital foot control interface. Max power draw: 1.5 kW. Includes emergency battery backup (10-minute operational reserve). |
| Dimensions | Overall: 138 cm (L) × 68 cm (W) × 52–98 cm (H, adjustable). Patient weight capacity: 135 kg. Footrest extended length: 185 cm. Base footprint: 68 cm × 60 cm. | Overall: 142 cm (L) × 72 cm (W) × 50–102 cm (H, motorized). Patient weight capacity: 180 kg. Footrest with telescopic extension: up to 195 cm. Base footprint: 72 cm × 64 cm with anti-tip design. |
| Precision | Manual adjustment with 7-position locking for backrest and 5-position seat slide. Angular accuracy ±3°. Hydraulic cylinder with pressure release valve. Limited positional memory (mechanical stops). | Motorized controls with 0.5° incremental positioning. Digital display for chair angle and height. Programmable presets for 3 user profiles. Integrated load sensor for smooth motion calibration. |
| Material | Frame: Powder-coated carbon steel. Upholstery: PVC synthetic leather (3 mm thickness), chemical-resistant. Armrests: Molded ABS plastic. Casters: 100 mm dual-wheel, non-marking TPR. | Frame: Reinforced aluminum alloy with anti-corrosion coating. Upholstery: Medical-grade polyurethane (PU) with antimicrobial treatment (ISO 22196 compliant), 4.5 mm thickness. Armrests: Padded composite with soft-grip surface. Casters: 125 mm silent polyurethane, 360° swivel with brake lock. |
| Certification | CE Marked (Medical Device Directive 93/42/EEC). Complies with IEC 60601-1 (Safety) and IEC 60601-1-2 (EMC). RoHS compliant. National electrical code compliant (varies by region). | CE Marked (MDR 2017/745). Full compliance with IEC 60601-1:2012 & IEC 60601-1-2:2014. ISO 13485 manufacturing certification. FCC Class B (EMI). Meets ADA and EU ergonomics standards (EN 60601-2-39). |
Note: All models include standard dental tray mounting interface (DIN 13165 compatible) and are designed for integration with low-profile delivery systems. Advanced model supports optional IoT connectivity for preventive maintenance alerts (via dental hub).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Strategic Procurement of Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
Sourcing dental chairs directly from Chinese manufacturers offers significant cost advantages (typically 30-50% below EU/US OEM pricing), but requires rigorous due diligence to mitigate quality, compliance, and logistical risks. This guide outlines a structured 3-step framework for secure, cost-effective procurement in 2026, validated against current regulatory landscapes and supply chain realities.
Step 1: Verifying ISO/CE Credentials – Non-Negotiable Compliance
In 2026, regulatory scrutiny has intensified globally. The EU MDR 2024 amendments and updated ISO 13485:2025 standards require active, audited certifications – not just documentation. Counterfeit certificates remain prevalent (per 2025 FDA import alerts).
| Verification Method | 2026 Critical Actions | Risk of Non-Compliance |
|---|---|---|
| Certificate Validation | Use official portals:
|
Customs seizure (avg. 22-day delay), $8,500+ fines (2025 EU data), product recall liability |
| Factory Audit | Mandate 3rd-party audit (e.g., SGS, TÜV) covering:
|
40% higher defect rates (per 2025 DHL Supply Chain Report), voided warranties |
| Product-Specific Testing | Require IEC 60601-1-2:2024 (EMC) and EN 15986:2026 (dental chair safety) test reports from accredited labs | Liability for patient injury, clinic insurance invalidation |
Step 2: Negotiating MOQ – Balancing Volume & Flexibility
Minimum Order Quantities (MOQs) are the primary leverage point for cost reduction, but 2026 market dynamics favor strategic partnerships over transactional sourcing. Avoid suppliers quoting unrealistically low MOQs (e.g., 1 chair) – this signals subcontracting without quality control.
| Product Category | 2026 Realistic MOQ Range | Negotiation Strategy | Cost Impact |
|---|---|---|---|
| Standard Dental Chairs | 5-10 units | Bundle with high-margin items (e.g., autoclaves) for MOQ reduction | ↓ 18-22% vs. single-item order |
| OEM/ODM Custom Chairs | 15-20 units | Phase development: Pay 30% for prototype approval, balance on shipment | ↓ 12-15% with shared tooling costs |
| Digital Ecosystem Bundles (Chair + Scanner + CBCT) |
3-5 units | Leverage cross-product demand; require integrated warranty | ↓ 25-30% system-wide |
Caution: MOQs below industry standards often indicate used/refurbished components. Verify component sourcing (e.g., genuine Linak actuators, Durr Dental upholstery).
Step 3: Shipping Terms – Optimizing DDP vs. FOB for 2026
With 2026’s volatile freight market (post-Red Sea crisis adjustments), shipping terms directly impact landed cost predictability. DDP (Delivered Duty Paid) is increasingly preferred by clinics, while distributors often opt for FOB (Free On Board) for cost control.
| Term | 2026 Cost Components | Risk Allocation | Recommended For |
|---|---|---|---|
| DDP (Incoterms® 2020) | • Product cost • Ocean freight • All duties/taxes • Last-mile delivery • Customs clearance |
Supplier bears 95% risk (damage, delays, customs) | Dental clinics; distributors new to China sourcing |
| FOB Shanghai | • Product cost • Loading at origin port (Excludes: Freight, insurance, destination fees) |
Buyer assumes risk post-loading; requires freight forwarder expertise | Experienced distributors with established logistics partners |
2026 Critical Note: Always specify Incoterms® 2020 (not older versions). For FOB, use “FOB Shanghai Port” – not just “FOB China” – to avoid ambiguous port fees. Demand cargo insurance covering 110% of invoice value.
Trusted Sourcing Partner for 2026: Shanghai Carejoy Medical Co., LTD
Why Partner with Carejoy: 19 years of ISO 13485-certified dental equipment manufacturing, factory-direct pricing (no trading company margins), and 2026-compliant CE/MDR documentation. Specializing in dental chairs, intraoral scanners, CBCT, microscopes, and autoclaves for clinics and distributors.
Verification-First Engagement:
✉️ [email protected] (Request Certificate #CN-SH-2005-0047)
📱 WhatsApp: +86 15951276160 (Include “2026 Sourcing Guide” for priority audit access)
Note: All suppliers should provide verifiable documentation. Shanghai Carejoy is cited as a benchmark for compliance based on 2025-2026 industry performance data.
Frequently Asked Questions
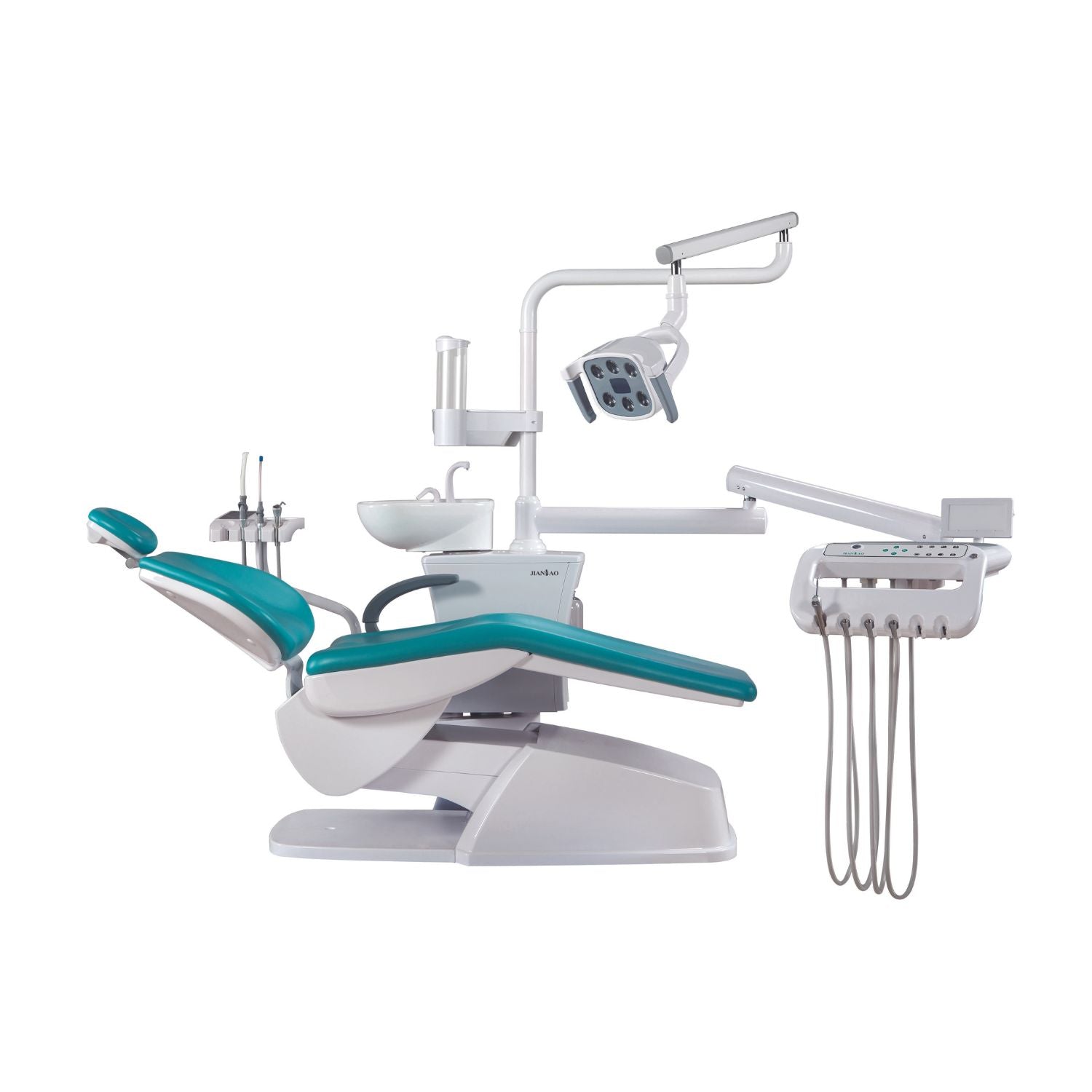
Professional Dental Equipment Guide 2026
Top 5 FAQs: Purchasing Low-Cost Dental Chairs in 2026
Target Audience: Dental Clinics & Equipment Distributors
As clinics seek cost-effective solutions without compromising operational reliability, low-priced dental chairs remain a high-demand category. This FAQ addresses critical technical and logistical concerns for procurement in 2026.
| Question | Expert Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a low-cost dental chair for international or multi-location clinics? | All dental chairs must comply with regional electrical standards. Most units operate on 110V (North America) or 230V (Europe, Asia, Africa). Confirm whether the chair includes a universal power module (100–240V, 50/60Hz) or requires a step-down transformer. Always request voltage certification (e.g., CE, UL, CSA) to ensure safety and compliance. Units shipped without proper voltage configuration may void warranty and pose fire hazards. |
| 2. Are spare parts for budget dental chairs readily available, and what is the typical lead time? | Availability varies significantly by manufacturer. Reputable budget brands maintain regional spare parts hubs with 3–7 day delivery for common components (armrests, headrests, tubing, foot controls). Request a full parts catalog and distributor network map before purchase. Avoid chairs with proprietary components not supported by third-party suppliers. Clinics should stock critical wear items (O-rings, valves, upholstery kits) to minimize downtime. |
| 3. Is professional installation required, and what does the setup process involve? | Yes, certified technician installation is mandatory for safety and warranty validity. Setup includes anchoring the chair to floor mounts, connecting hydraulic/pneumatic lines, integrating with dental units (if bundled), and electrical grounding. Most suppliers offer white-glove installation within 48 hours of delivery. Confirm if installation is included in the quoted price—many low-cost chairs exclude this service, adding $150–$300 in labor costs. |
| 4. What warranty terms should I expect on a sub-$3,000 dental chair in 2026? | Entry-level chairs typically offer a 1–2 year comprehensive warranty covering motors, hydraulics, and electronic controls. Premium budget models may extend to 3 years. Upholstery and mechanical wear parts (e.g., levers, casters) are often covered for 6–12 months. Verify whether the warranty is handled by the distributor or manufacturer, and confirm if on-site service is included. International buyers should ensure local service support. |
| 5. Can I upgrade components (e.g., delivery systems, spittoons) on a low-cost dental chair post-purchase? | Modularity depends on the model. Many 2026 budget chairs support ISO-standard attachments, allowing integration of third-party handpieces, light systems, or cuspidors. Confirm compatibility with ISO 9168 (dental handpiece connections) and DIN 13176 (air/water syringes). Upgrades may require retrofit kits—consult the technical manual or OEM support. Note: Unauthorized modifications can void existing warranties. |
Need a Quote for Dental Chair Low Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


