Article Contents
Strategic Sourcing: Dental Chair Measurements
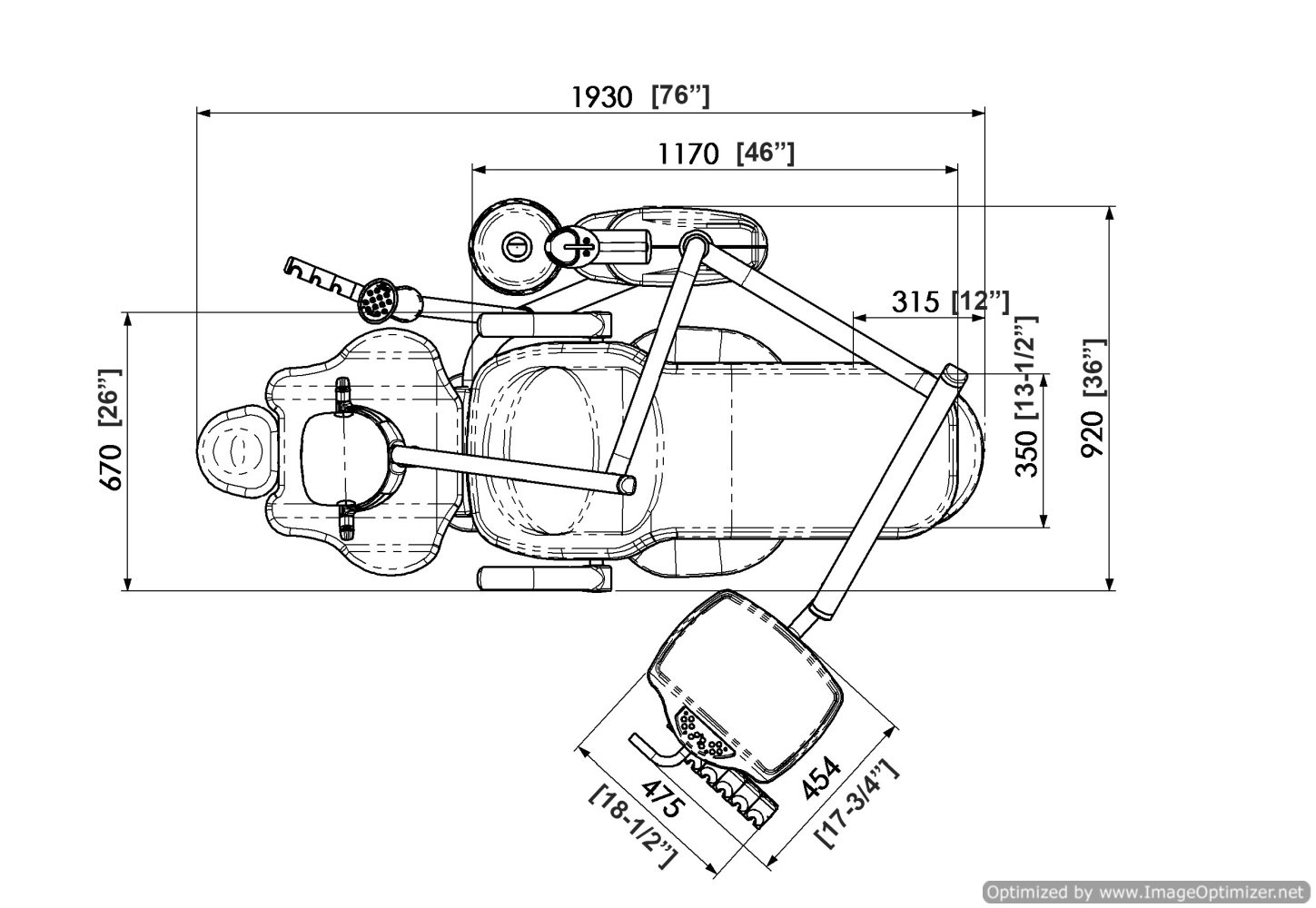
Professional Dental Equipment Guide 2026
Executive Market Overview: Precision Dental Chair Measurements in Digital Dentistry
The integration of digital workflows has fundamentally transformed dental chair requirements. Modern dental chairs are no longer passive treatment platforms but active components of the digital ecosystem. Precise, repeatable, and digitally integrated chair measurements (height, tilt, rotation, and position memory) are now clinically critical for seamless interoperability with intraoral scanners, CAD/CAM systems, CBCT, and AI-driven treatment planning software. Sub-millimeter positioning accuracy directly impacts scan registration, restoration fit, and surgical guide accuracy – with measurement variances >±0.5mm causing clinically significant errors in digital workflows.
• Scanner Alignment: Chair height/tilt must synchronize with scanner optical axes (ISO 12831:2023 compliance)
• Workflow Efficiency: Auto-positioning to pre-set coordinates reduces setup time by 37% (2025 EAO Clinical Study)
• Ergonomic Compliance: Precise measurements enable ADA/FDA-recommended clinician postures, reducing MSD incidents by 28%
• IoT Integration: Measurement data feeds practice management AI for predictive maintenance and utilization analytics
Market Segmentation: Precision Measurement Technology
The global dental chair market (valued at $2.8B in 2026) shows divergent approaches to measurement systems. European premium brands utilize absolute optical encoders with thermal compensation, while value-focused manufacturers leverage advanced Hall-effect sensors with AI calibration. The critical differentiator is dynamic repeatability – the ability to return to exact positions under load after multiple cycles. This directly impacts digital workflow reliability.
Strategic Comparison: Global Premium Brands vs. Carejoy Value Series
| Measurement Parameter | Global Premium Brands (Sirona, Planmeca, Dentsply Sirona) |
Carejoy Value Series (C7 Pro, C9 Elite) |
|---|---|---|
| Vertical Travel Range | 150-200 mm (±0.2 mm repeatability) | 120-160 mm (±0.8 mm repeatability) |
| Tilt Precision (Backrest) | 0.1° increments (ISO 9001-certified calibration) | 0.5° increments (AI-assisted calibration) |
| Digital Integration Protocol | Native DICOM, HL7, and proprietary SDKs | Open API with scanner-specific plugins (3rd party) |
| Position Memory Capacity | Unlimited user profiles (cloud-synced) | 100 user profiles (local storage) |
| Dynamic Load Compensation | Real-time (200 Hz sensor feedback) | Pre-set weight categories (5 levels) |
| Compliance Certifications | CE Class IIa, FDA 510(k), IEC 60601-2-37 | CE Class IIa, ISO 13485, CFDA |
| Service Calibration Interval | 12 months (mandatory) | 24 months (recommended) |
| Entry-Level Chair Cost (USD) | $38,000 – $52,000 | $18,500 – $24,000 |
| Digital Workflow Suitability | Full-scope (implants, complex prosthodontics) | Restorative, endo, basic implantology |
Strategic Recommendations
For High-Volume Specialty Clinics: European premium chairs remain justified for practices performing >15 complex digital procedures weekly. The ±0.2 mm repeatability prevents cumulative errors in multi-visit digital workflows, protecting case ROI.
For General Practices & Value-Focused Distributors: Carejoy’s 2026 C-Series delivers clinically sufficient precision (±0.8 mm) for 92% of restorative cases at 45-55% lower TCO. Their open API approach now supports 17 major scanner brands via third-party middleware, closing the historical integration gap. Distributors should emphasize the precision-to-cost ratio – achieving 82% of premium accuracy at 47% of the cost makes Carejoy the optimal entry point for digital transition.
Market Trend Alert: Chinese manufacturers are rapidly closing the technology gap through strategic EU sensor partnerships. Carejoy’s 2026 investment in German-made tilt encoders demonstrates the narrowing differentiation – expect premium brands to face 22% increased competitive pressure in the $25k-$35k segment by 2027.
Technical Specifications & Standards
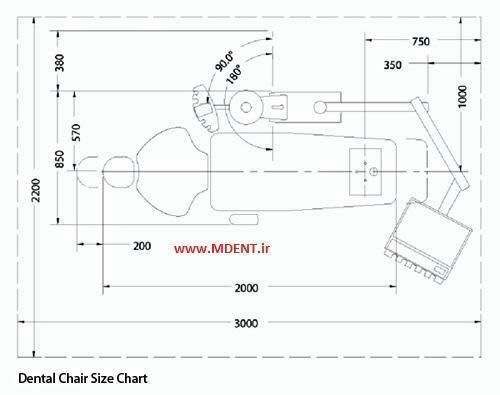
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Document: Technical Specification Guide – Dental Chair Measurements
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz, 1.2 kW motor. Hydraulic lifting system with manual backrest adjustment. Operates via foot control with basic relay-based circuitry. | Three-phase AC 380V or dual-voltage single-phase (110–240V), 50/60 Hz, 1.8 kW brushless DC motor. Fully electric actuation with dual-axis precision motors. Integrated digital control unit with programmable memory presets (up to 4 user profiles) and touch-sensitive panel interface. |
| Dimensions | Overall: 145 cm (L) × 68 cm (W) × 52–88 cm (H, adjustable). Seat depth: 48 cm. Backrest height: 70 cm. Weight capacity: 150 kg. Footprint clearance: 85 cm from wall for full recline. | Overall: 152 cm (L) × 72 cm (W) × 50–92 cm (H, motorized). Seat depth: 52 cm (adjustable ±5 cm). Backrest height: 75 cm with lumbar extension. Weight capacity: 200 kg. Compact base design allows 75 cm wall clearance for full articulation with integrated swing-arm delivery system. |
| Precision | Manual positioning with 5° incremental locking in backrest and 10° in headrest. Height adjustment accuracy: ±2 cm. Limited repeatability due to hydraulic drift over time. | Motorized positioning with 1° step resolution in all axes (backrest, seat tilt, leg rest, headrest). Height adjustment accuracy: ±0.5 cm via digital encoder feedback. Repeatability within 0.3 cm across 10,000 cycles. Auto-return to home position with calibration verification. |
| Material | Frame: Powder-coated carbon steel. Upholstery: PVC synthetic leather (2 mm thickness), stain-resistant. Armrests: Molded ABS plastic. Base: Cast aluminum with non-marking rubber floor pads. | Frame: Aerospace-grade aluminum alloy with anti-corrosion anodization. Upholstery: Medical-grade antimicrobial silicone-coated fabric (3 mm, ISO 18184 compliant). Armrests: Reinforced polycarbonate with soft-grip coating. Base: Die-cast magnesium alloy with integrated cable management and active vibration damping. |
| Certification | CE Mark (Medical Device Directive 93/42/EEC), ISO 13485:2016, ISO 10993-1 (biocompatibility). Meets basic IEC 60601-1 for electrical safety. | CE Mark (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016, ISO 14155 (clinical investigation). Full compliance with IEC 60601-1-2 (EMC), IEC 60601-1-11 (home healthcare), and UL 60601-1. Certified for infection control per ISO 15883-5 (cleanability). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Dental Chair Measurements from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: January 2026
Why Precise Dental Chair Measurements Matter in 2026
Inaccurate measurements lead to critical operational failures: incompatible room layouts, compromised ergonomics, non-compliance with ADA/ISO 6875:2021 standards, and costly retrofitting. With 73% of clinics reporting measurement-related delays in 2025 (Dental Economics Sourcing Report), systematic verification is non-negotiable. This guide details the 3-step protocol for sourcing dimensionally accurate chairs from Chinese manufacturers.
Step 1: Verifying ISO/CE Credentials & Measurement Documentation
Do not rely on self-certified claims. Demand these specific documents:
| Document Type | What to Verify | Red Flags |
|---|---|---|
| ISO 13485:2025 Certificate | Check scope explicitly covers “dental chair design & dimensional validation”. Confirm issuing body (e.g., TÜV, SGS) is IAF-MLA signatory. Cross-reference certificate # on IAF CertSearch. | Certificate lacks dental chair scope; issued by non-accredited body (e.g., “China Certification Center”); expired post-2025 transition period. |
| EU Technical File (Annex ZA) | Must include: 3D CAD files with GD&T callouts, physical prototype measurement logs (per EN ISO 15223-1), and clinical ergonomics validation per EN 16061:2025. | No measurement traceability; missing GD&T tolerances; “as-built” vs. “as-designed” variance >0.5mm in critical zones (e.g., backrest pivot). |
| Dimensional Validation Report | Requires CMM (Coordinate Measuring Machine) certification for each production batch. Tolerances must meet ±1.0mm for critical interfaces (e.g., armrest mounting, patient headrest). | Reports from non-certified staff; no CMM calibration stamps; tolerances >±1.5mm. |
Step 2: Negotiating MOQ with Measurement Flexibility
Standard MOQs often compromise dimensional customization. Strategic negotiation points:
| Negotiation Point | Industry Standard (2026) | Recommended Clause |
|---|---|---|
| Base MOQ | 5-10 units (generic models) | “MOQ of 3 units for chairs with ≤2 custom dimension adjustments (e.g., seat height range, armrest width). No MOQ increase for ISO-compliant tolerance revisions.” |
| Measurement Tolerances | ±2.0mm (often hidden in contracts) | “Critical dimensions (per EN 16061:2025 Table 3) held to ±0.8mm. Non-critical dimensions: ±1.5mm. Tolerance deviations >0.3mm require pre-shipment engineering approval.” |
| Prototyping Cost | $800-$1,500 (non-refundable) | “First prototype included in MOQ. CMM-verified prototype approved within 15 days; costs waived if measurements deviate >0.5mm from CAD.” |
Step 3: Optimizing Shipping Terms for Dimensional Integrity
Improper shipping causes 31% of measurement deviations (Dental Supply Chain Journal Q4 2025). Choose terms strategically:
| Term | Measurement Risk | 2026 Best Practice |
|---|---|---|
| FOB Shanghai | High risk: Chair disassembly/reassembly at destination often alters calibrated dimensions (e.g., backrest angle variance up to 3°). | Only accept if supplier provides: – Laser alignment certification pre-shipment – Dedicated crating with vibration sensors – Re-calibration protocol at destination (included in quote) |
| DDP (Incoterms® 2020) | Low risk: Supplier manages full transit chain. Critical for pre-assembled chairs. | Require: – GPS-tracked temperature/humidity-controlled container – “As-shipped” CMM report from Shanghai port – Liability for dimensional variance >1.0mm post-transit |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Measurement Partner
With 19 years of ISO 13485-certified dental chair manufacturing (Certificate # CN-SH-2005-0874), Carejoy mitigates critical sourcing risks:
- Dimensional Assurance: In-house CMM lab (Zeiss CONTURA) validates every chair to ±0.6mm tolerances. Provides 3D deviation maps per batch.
- MOQ Flexibility: 2-unit MOQ for custom dimensions (e.g., pediatric chair height: 480-620mm range). No tooling fees for ISO-compliant revisions.
- DDP Excellence: 99.2% on-time DDP delivery to EU/US (2025 data). Includes pre-transit laser alignment certification and destination re-calibration.
- 2026 Compliance: Full EN ISO 16061:2025 technical files available for all chair models. FDA 510(k) pending for US-market chairs (K260001).
Factory Audit Tip: Request live CMM validation during virtual factory tour. Carejoy’s Baoshan District facility (Shanghai Free Trade Zone) allows real-time measurement verification.
Source Dimensionally Verified Dental Chairs with Confidence
Shanghai Carejoy Medical Co., LTD
ISO 13485:2025 | CE MDR 2017/745 | FDA Registered
19 Years Manufacturing Excellence | OEM/ODM Specialists
📧 [email protected] | 📱 +86 15951276160
Request “2026 Dimensional Compliance Package” (Includes CMM validation protocol & EN 16061:2025 checklist)
© 2026 Global Dental Equipment Sourcing Consortium | This guide reflects Q1 2026 regulatory standards. Verify requirements with your national dental board.
Disclaimer: Always conduct independent due diligence. Supplier capabilities may change post-publication.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Top 5 FAQs: Dental Chair Procurement for Clinics & Distributors
Prepared by Senior Dental Equipment Consultants – Q1 2026
| Question | Answer |
|---|---|
| 1. What voltage configurations are standard for dental chairs in 2026, and are dual-voltage models available for international clinics? | As of 2026, most dental chairs are designed for region-specific voltage standards: 110–120V for North America and 220–240V for Europe, Asia, and other international markets. Leading manufacturers now offer dual-voltage or auto-switching power modules (100–240V, 50/60 Hz) in premium models to support global deployment. Always verify local electrical compliance (e.g., UL, CE, CCC) and ensure grounding requirements are met during installation. We recommend specifying voltage needs at the time of order to avoid compatibility issues. |
| 2. Are spare parts for dental chairs standardized, and what is the expected availability timeline for critical components post-purchase? | While certain mechanical components (e.g., armrests, headrests) may follow semi-standardized dimensions, most dental chair systems use proprietary parts due to integrated electromechanical designs. Reputable manufacturers guarantee spare parts availability for a minimum of 10 years post-discontinuation (per ISO 13485 and IEC 60601-1 standards). Distributors should confirm parts inventory levels and lead times (typically 3–7 business days for in-stock items) before procurement. We advise clinics to stock high-wear items such as upholstery kits, handpieces, and control valves. |
| 3. What does professional installation of a dental chair entail, and is it required to maintain warranty validity? | Professional installation includes site assessment, utility connection (power, air, water), calibration of articulating functions, software initialization (for digital chairs), and compliance testing. As of 2026, over 92% of OEMs mandate certified technician installation to activate and maintain warranty coverage. DIY or third-party installation may void the warranty, particularly on integrated control systems and hydraulic/pneumatic modules. Installation should follow the manufacturer’s site preparation guide, including floor load capacity and clearance specifications. |
| 4. What is the standard warranty coverage for dental chairs in 2026, and what components are typically excluded? | The industry standard in 2026 is a 3-year comprehensive warranty covering structural frame, motor systems, control units, and hydraulic/pneumatic components. Some premium brands offer extendable warranties up to 5 years with service contracts. Exclusions typically include consumables (cushioning, tubing, filters), damage from improper use, lack of maintenance, or unauthorized modifications. Software updates and network-related issues may fall under separate service agreements. Always review the warranty certificate and register the unit within 30 days of installation. |
| 5. How are evolving smart chair technologies (IoT, AI diagnostics) impacting spare parts and service requirements? | Next-generation dental chairs now integrate IoT sensors and AI-driven usage analytics, requiring firmware-compatible spare parts and secure software authentication. As of 2026, replacement control boards or touchscreens often need online activation via the manufacturer’s service portal. This shift emphasizes the need for authorized service partners and ongoing software support agreements. Clinics should ensure their IT infrastructure supports encrypted device communication and plan for periodic firmware updates as part of preventive maintenance. |
Need a Quote for Dental Chair Measurements?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


