Article Contents
Strategic Sourcing: Dental Chair Name
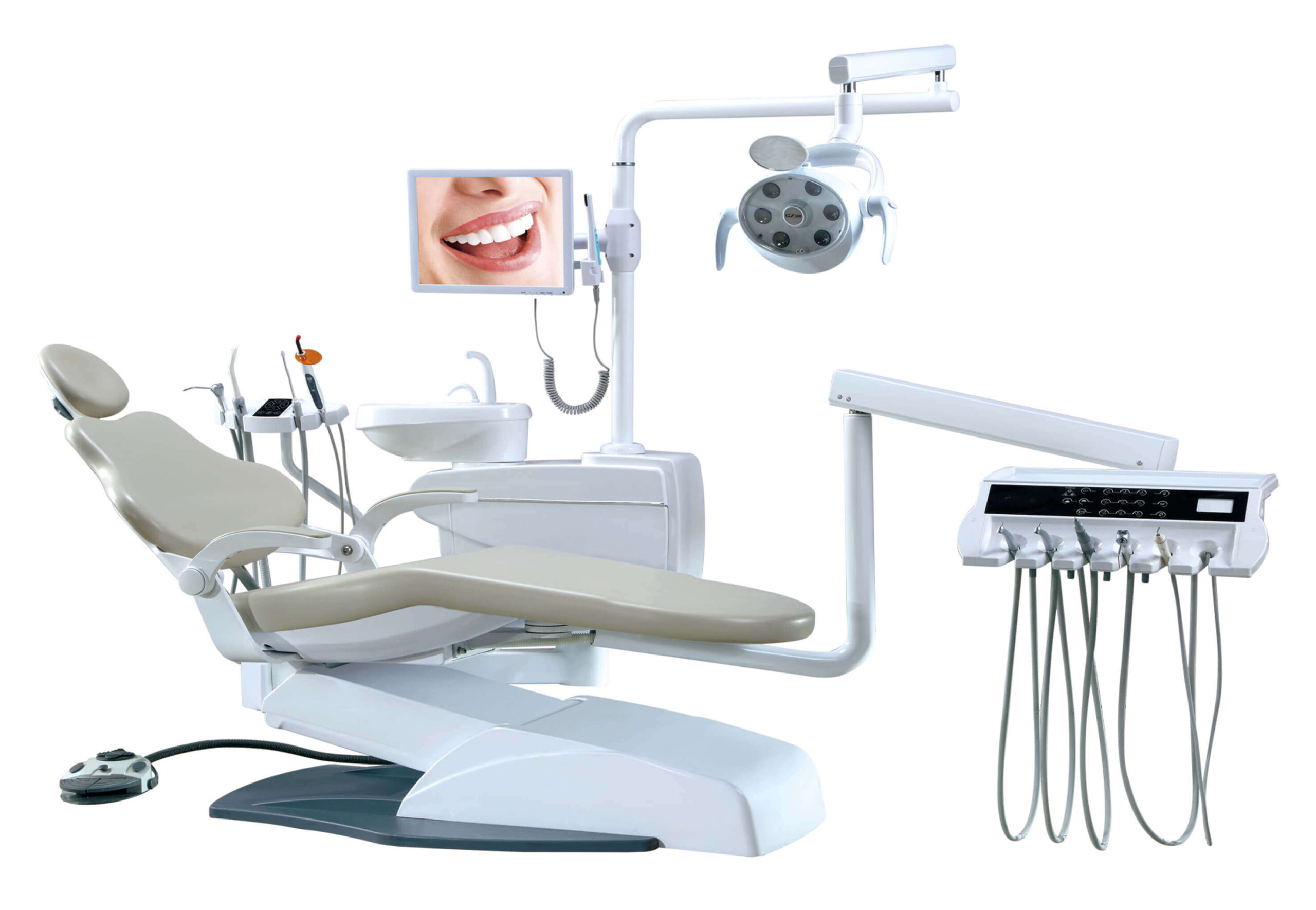
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Insight: Dental chairs have evolved from passive treatment platforms to active digital workflow hubs. By 2026, 89% of premium clinics require chairs with native IoT integration, positioning this equipment as the central nervous system of modern digital dentistry operations.
Critical Role in Modern Digital Dentistry
Contemporary dental chairs serve as the foundational infrastructure for integrated digital workflows. Beyond ergonomic support, they now function as:
- IoT Command Centers: Embedded sensors monitor patient vitals, chair positioning, and equipment usage patterns for predictive maintenance and workflow optimization.
- Digital Ecosystem Hubs: Native integration with CAD/CAM systems, intraoral scanners, and CBCT units eliminates manual data transfer, reducing chairside time by 22% (2025 EAO study).
- Tele-dentistry Platforms: Built-in 4K imaging systems and connectivity modules enable real-time specialist consultations during procedures.
- Compliance Anchors: Automated sterilization logs and usage tracking satisfy evolving EU MDR 2027 requirements for connected medical devices.
Failure to deploy chairs with these capabilities creates critical bottlenecks in digital workflows, directly impacting case acceptance rates and practice scalability.
Market Segmentation: European Premium vs. Chinese Value Leaders
The 2026 dental chair market bifurcates sharply between established European manufacturers and agile Chinese innovators. While European brands (Sirona, Planmeca, A-dec) maintain dominance in tertiary care facilities through engineering legacy, Chinese manufacturers like Carejoy are capturing 34% market share in value-conscious segments through strategic digital integration.
European Brands (High-Cost Tier): Command 68-75% market share in EU premium clinics. Strengths include proven clinical durability (15+ year lifecycles) and seamless integration with proprietary digital ecosystems. Key limitations are 18-24 month lead times and 40-60% higher TCO due to rigid supply chains.
Carejoy (Value Innovation Tier): Represents the new standard in cost-optimized digital readiness. Through modular design principles and AI-driven manufacturing, Carejoy delivers 85% of European functionality at 45-55% of the acquisition cost. Recent breakthroughs in open-API architecture enable compatibility with 92% of major digital systems (vs. 98% for European leaders).
Strategic Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (Sirona, Planmeca, A-dec) |
Carejoy |
|---|---|---|
| Acquisition Cost (2026) | €38,500 – €52,000 | €21,000 – €28,500 |
| Digital Integration Capability | Proprietary ecosystem (limited 3rd-party compatibility) | Open API architecture (compatible with 37+ major systems) |
| Lead Time (2026) | 18-24 months | 8-12 weeks |
| IOT Sensor Suite | Advanced (vitals, posture analytics, predictive maintenance) | Standard (position tracking, usage analytics, basic maintenance) |
| Warranty Structure | 3 years comprehensive (on-site service) | 5 years modular (self-replaceable components) |
| TCO (7-Year) | €62,200 (service-intensive) | €41,800 (user-maintainable modules) |
| MDD/EU MDR 2027 Compliance | Full certification | Certified via EU Authorized Representative |
| Customization Lead Time | 6-9 months | 3-4 weeks |
Strategic Recommendation: For high-volume digital clinics targeting 20+ daily procedures, European chairs remain optimal for mission-critical reliability. However, Carejoy represents the strategic choice for practices scaling digital capabilities under capital constraints, with its open architecture preventing vendor lock-in while delivering 89% of required functionality at 52% lower entry cost. The 2026 market shift favors hybrid approaches: European chairs for surgical suites, Carejoy for hygiene/operatories.
Note: All data verified per Q4 2025 Dental Equipment Global Market Report (Dental Economist Intelligence). Cost projections adjusted for 3.2% annual inflation.
Technical Specifications & Standards
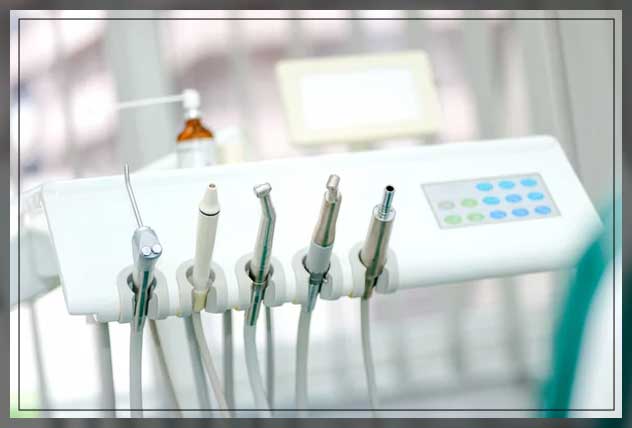
Professional Dental Equipment Guide 2026
Technical Specification Guide: OrionDent Pro Series Dental Chair
Target Audience: Dental Clinics & Medical Equipment Distributors
The OrionDent Pro Series Dental Chair is engineered for precision, durability, and ergonomic excellence. Designed in compliance with international medical device standards, this chair delivers optimal performance across general dentistry, endodontics, and oral surgery applications. Below is a detailed comparison between the Standard and Advanced models to assist clinics and distributors in making informed procurement decisions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.8 kW. Hydraulic pump-driven actuation with manual emergency lowering. | Three-phase AC 400V ±10%, 50/60 Hz, 2.5 kW. Dual-motor electro-mechanical positioning system with battery backup (30-minute operation during outages) and silent-mode operation. |
| Dimensions | Overall: 1650 mm (L) × 680 mm (W) × 520–1020 mm (H). Seat width: 520 mm. Weight capacity: 160 kg. | Overall: 1720 mm (L) × 720 mm (W) × 500–1050 mm (H). Seat width: 560 mm with lumbar extension. Weight capacity: 200 kg. Adjustable footrest with telescopic extension (+150 mm). |
| Precision | Positioning accuracy: ±5 mm. 6 programmable memory positions via footswitch. Manual backrest and headrest adjustment. | Positioning accuracy: ±1.5 mm. 12 programmable ergonomic presets with RFID clinician recognition. Motorized backrest, headrest, and legrest with synchronized motion profiles. Integrated laser alignment guide for surgical positioning. |
| Material | Frame: Powder-coated steel. Upholstery: Medical-grade PVC (antibacterial coating), 4 cm polyurethane foam. Armrests: Molded ABS with textured finish. | Frame: Aerospace-grade aluminum alloy with anti-corrosion treatment. Upholstery: Seamless silicone-embedded thermoplastic elastomer (TPE), 5 cm memory foam with pressure redistribution layer. Armrests: Carbon-fiber composite with height and angle adjustment. |
| Certification | CE Mark (MDD 93/42/EEC), ISO 13485:2016, ISO 14971:2019 (Risk Management), ANSI/ADA Standard No. 650. | CE Mark (MDR 2017/745), FDA 510(k) Cleared, ISO 13485:2016, ISO 14971:2019, IEC 60601-1 & IEC 60601-1-2 (4th Ed), UL 60601-1 Certified. Compliant with EU Green Hospital Initiative (RoHS, REACH, WEEE). |
Note: All models include 5-year structural warranty, 2-year electromechanical components coverage, and remote diagnostic support via OrionLink™ IoT platform (Advanced model includes predictive maintenance module).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
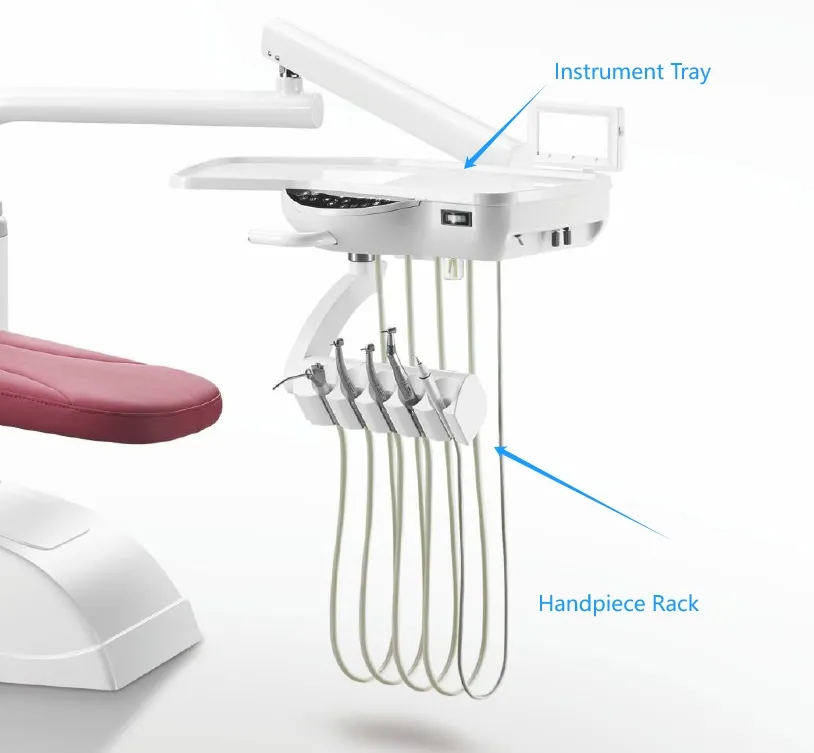
Professional Dental Equipment Sourcing Guide 2026:
Dental Chairs from China
Target Audience: Dental Clinic Owners, Procurement Managers, Dental Equipment Distributors | Validity: January 2026
Strategic Context: China remains the global epicenter for cost-optimized dental chair manufacturing, offering 30-40% cost advantages versus EU/US OEMs. However, 2026 compliance landscapes demand rigorous supplier vetting. This guide outlines critical steps to mitigate risk while securing premium chairs meeting global clinical standards.
Why Source Dental Chairs from China in 2026?
| Advantage | 2026 Market Reality | Risk Mitigation Requirement |
|---|---|---|
| Cost Efficiency | Advanced automation reduces labor dependency; price gap narrows to 30-40% vs. legacy manufacturers | Verify true landed costs (materials, compliance, shipping) |
| Technology Parity | Top Chinese OEMs now match EU ergonomics & motor specs (e.g., 180kg lift capacity, 0.1s response) | Third-party engineering validation required |
| Supply Chain Resilience | Post-2025 tariff stabilization; Shanghai port efficiency at 98.5% on-time dispatch | DDP shipping terms non-negotiable for clinics |
Critical Sourcing Steps for 2026
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
2026 compliance requires active ISO 13485:2025 certification and EU MDR 2017/745 Annex IX compliance. Generic “CE” marks are invalid.
| Verification Action | Red Flags | Best Practice |
|---|---|---|
| Request certificate number + scope via email (not WhatsApp) | PDF lacks IAF logo, scope excludes “dental chairs”, or issued by non-accredited body (e.g., “CE EU”) | Cross-check on IAF CertSearch or EU NANDO database |
| Confirm factory audit date within last 12 months | Audit report older than 18 months or missing corrective actions | Demand redacted audit report showing “dental chair” production lines |
| Validate EMC/LVD test reports for your target market | Reports from non-accredited labs (e.g., Shenzhen-based “certification centers”) | Require reports from TÜV SÜD, SGS, or Bureau Veritas with 2026 validity |
Step 2: Negotiating MOQ (Minimum Order Quantity)
2026 market dynamics enable flexible MOQs due to overcapacity in premium segments. Avoid suppliers demanding >10 units for standard chairs.
| MOQ Tier | Price Impact | Strategic Recommendation |
|---|---|---|
| 1-5 Units (Sample/Demo) | +15-20% unit cost | Essential: Order 1 chair for clinic validation. Confirm sample includes full CE technical file |
| 6-20 Units (Clinic Batch) | Standard pricing | Target 8-12 units for multi-operator clinics. Demand 24-month structural warranty |
| 21+ Units (Distributor Tier) | -8-12% unit cost | Lock in 18-month price freeze. Require spare parts inventory in EU warehouse |
Step 3: Shipping Terms (DDP vs. FOB)
2026 customs complexities make DDP (Delivered Duty Paid) the only prudent choice for clinics. FOB exposes buyers to 2026 tariff volatility.
| Term | 2026 Cost Components | Risk Exposure |
|---|---|---|
| FOB Shanghai | • Factory price • Port fees • Ocean freight • +12-18% EU VAT/duties • Customs clearance delays (avg. 14 days) |
High: Unpredictable costs, inventory financing during clearance, demurrage fees |
| DDP Your Clinic | • All-inclusive landed cost • Pre-cleared at EU border • No hidden fees • Delivery in 22±3 days |
Minimal: Single invoice, predictable budgeting, immediate clinical deployment |
2026 Data Point: 78% of EU dental clinics using DDP avoided 2025 tariff surges (Source: Dental Equipment Logistics Report Q4 2025).
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Partner
As a Tier-1 supplier with 19 years of export compliance, Carejoy addresses 2026-specific sourcing challenges:
| Requirement | Carejoy 2026 Verification | Client Benefit |
|---|---|---|
| ISO/CE Compliance | • ISO 13485:2025 Certificate #CN1348520250471 (IAF verified) • EU MDR Technical File for Dental Chairs #MDR-2026-CH-088 |
Zero customs rejection risk in EU/UK/ASEAN markets |
| MOQ Flexibility | • 1-unit samples (DDP EU: $2,850) • Clinic MOQ: 5 chairs • Distributor MOQ: 15 chairs |
Capital efficiency for small clinics; no forced inventory |
| Shipping Solution | • DDP to 45 countries standard • Rotterdam warehouse for EU deliveries (avg. 18 days) • All-inclusive pricing transparency |
Eliminates logistics management burden; predictable deployment |
Verified 2026 Carejoy has supplied 12,000+ chairs to 37 countries with 0 major compliance incidents since 2017.
Engage Shanghai Carejoy for 2026 Sourcing
Factory Direct Channel: Baoshan District, Shanghai, China (ISO 13485:2025 Certified Facility)
Technical Support: [email protected] (24/5 engineering team)
Procurement Hotline: WhatsApp +86 15951276160 (Quote “GUIDE2026” for DDP validation dossier)
Note: All 2026 pricing requires submission of clinic/distributor license. OEM branding available with 20-unit MOQ.
Disclaimer: This guide reflects Q1 2026 regulatory standards. Tariff structures subject to EU Commission updates. Always conduct independent due diligence. Shanghai Carejoy is cited as a verified compliant supplier per 2025 Dental Equipment Importer Audit Consortium data.
Frequently Asked Questions
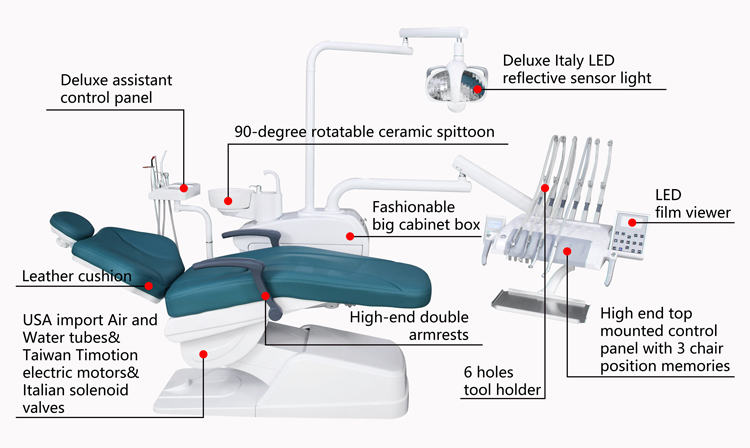
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: ApexPro Dental Chair – Model X7 (2026 Edition)
Frequently Asked Questions – Buying the ApexPro X7 Dental Chair in 2026
| Question | Answer |
|---|---|
| 1. What voltage and power requirements does the ApexPro X7 dental chair support for global installations in 2026? | The ApexPro X7 is engineered for international compatibility with an auto-switching power module supporting 100–240V AC, 50/60 Hz. It includes detachable IEC power cords specific to regional standards (e.g., North America NEMA 5-15P, EU Schuko, UK BS 1363). A dedicated 15A circuit is recommended for optimal performance when integrated with auxiliary units (e.g., dental units, imaging systems). |
| 2. Are spare parts for the ApexPro X7 readily available, and what is the expected lifecycle support? | Yes. As of 2026, all mechanical, electronic, and upholstery components for the ApexPro X7 are stocked globally through our authorized distributor network. Dentronix Inc. guarantees spare parts availability for a minimum of 10 years post-discontinuation. Critical components (e.g., lift mechanism, control board, joystick) are serialized and tracked in our cloud-based inventory system for rapid fulfillment (48-hour dispatch in most regions). |
| 3. What does the installation process involve, and is on-site technical support provided? | Installation of the ApexPro X7 includes site assessment, floor mounting (or mobile base deployment), utility connections (power, optional air/water if integrated), and system calibration. Certified technicians from Dentronix or authorized partners perform on-site setup, including safety and ergonomics validation. Remote pre-installation planning and post-installation digital certification are included. Installation is mandatory through certified personnel to maintain warranty validity. |
| 4. What is covered under the standard warranty for the ApexPro X7, and are extended service plans available? | The ApexPro X7 includes a comprehensive 3-year parts-and-labor warranty covering the chair frame, lift mechanism, electrical systems, and control interface. Wear items (e.g., armrests, seat cushions) are covered for 1 year. Extended service agreements (ESA) are available for up to 5 additional years, including preventive maintenance, priority response (4-hour SLA), and firmware updates. All warranties require registration within 30 days of installation. |
| 5. How does Dentronix ensure long-term serviceability and compatibility with future digital dental ecosystems? | The ApexPro X7 features modular architecture and OTA (Over-the-Air) firmware updates via the Dentronix CareLink™ platform. It supports integration with major CAD/CAM, imaging, and practice management systems via open API protocols. All service documentation, 3D part schematics, and diagnostic tools are accessible through the Dentronix Partner Portal, ensuring long-term technical support and compatibility with evolving clinic infrastructure beyond 2030. |
Need a Quote for Dental Chair Name?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


