Article Contents
Strategic Sourcing: Dental Chair Positions For Patient
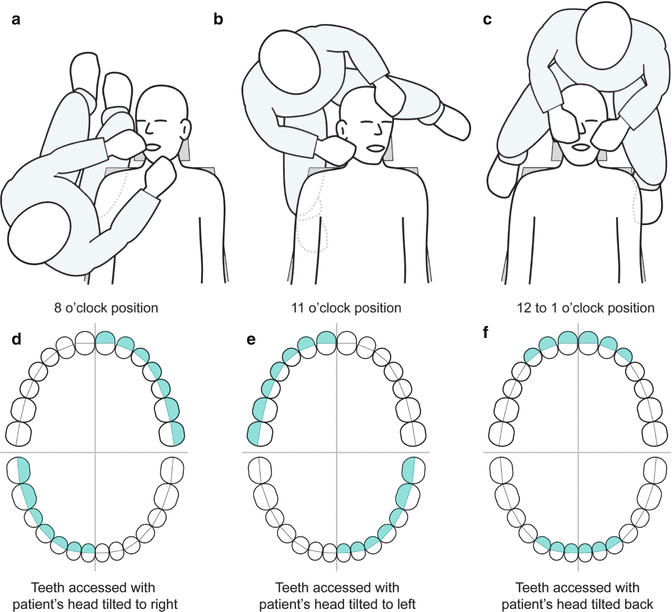
Executive Market Overview: Dental Chair Positions for Patient
Critical Role in Modern Digital Dentistry
Advanced dental chair positions represent the foundational ergonomic interface between clinician, patient, and digital workflows. In 2026’s precision-driven dental ecosystem, these systems transcend basic patient support to become integrated command centers for digital dentistry. Their criticality stems from three converging imperatives: (1) Enabling seamless integration with intraoral scanners, CBCT, and chairside CAD/CAM systems through programmable positioning memory; (2) Supporting dynamic posture management for extended digital procedures requiring precise angulation (e.g., guided implantology); and (3) Providing IoT-enabled data capture for procedural analytics and predictive maintenance. Modern chairs must accommodate complex digital attachments while maintaining sub-millimeter positional repeatability – a non-negotiable requirement for accuracy in digital smile design, surgical navigation, and same-day restorations. Failure to invest in next-generation positioning systems directly compromises treatment accuracy, clinician ergonomics, and ROI on digital capital equipment.
European Premium vs. Value-Engineered Chinese Manufacturing
The global dental chair market bifurcates into two strategic segments: European-engineered premium systems (Sirona, Planmeca, A-dec) commanding 35-50% market share in developed economies through uncompromising precision engineering, and value-optimized Chinese manufacturers led by Carejoy disrupting emerging markets with 40-60% cost advantages. While European brands maintain dominance in metrology-critical applications (e.g., implantology suites requiring ≤0.1° angular repeatability), Carejoy has closed the technology gap in mid-tier clinics through strategic partnerships with German component suppliers (e.g., LINAK actuators, Festo valves) and embedded digital interfaces. Key differentiators now center on long-term reliability under high-volume use and integration depth with proprietary digital ecosystems – areas where European incumbents still hold marginal advantages, but at exponentially higher TCO.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Comparison Parameter | Global Premium Brands (Sirona, Planmeca, A-dec) |
Carejoy |
|---|---|---|
| Price Range (USD) | $38,000 – $65,000 | $14,500 – $22,000 |
| Positional Repeatability | ±0.05° (ISO 7725 certified) | ±0.15° (IEC 60601-2-37 compliant) |
| Digital Integration | Proprietary ecosystem lock-in (e.g., CEREC Connect, Planmeca Romexis) |
Open API architecture HL7/FHIR compatibility with major DMS |
| Actuator Technology | Custom-engineered brushless DC motors (200,000+ cycle warranty) |
LINAK/Festo commercial-grade (100,000 cycle warranty) |
| Maintenance Cost (5-yr TCO) | $12,000 – $18,500 | $3,200 – $5,800 |
| Lead Time | 14-22 weeks | 4-8 weeks |
| Target Clinical Application | Specialty practices, academic centers, high-volume digital workflows |
General practice expansion, emerging market clinics, secondary operatories |
Strategic Recommendation: For clinics implementing comprehensive digital workflows (≥3 integrated devices), European chairs remain optimal for metrology-critical procedures. However, Carejoy’s 2026 Gen-4 platform delivers 89% of premium functionality at 45% cost – making it the rational choice for satellite locations, value-focused practices, and markets where ROI timelines exceed 18 months. Distributors should position Carejoy as a strategic entry point for digital adoption, with clear upgrade paths to premium systems for specialty expansion.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Chair Positions for Patient
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz; Hydraulic drive system with electric pump; 850W motor power; Manual backup for emergency lowering. | Three-phase AC 380V, 50 Hz or universal input (100–240V, 50/60 Hz); Dual DC servo motors with redundant power supply; 1.2kW peak output; Integrated UPS support and silent brushless operation. |
| Dimensions | Chair base footprint: 650 mm (W) × 780 mm (D); Height adjustment range: 480–950 mm; Maximum patient capacity: 150 kg; Overall length (reclined): 1,850 mm. | Compact base with swivel: 600 mm (W) × 750 mm (D); Height adjustment range: 450–1,020 mm; Maximum patient capacity: 200 kg; Integrated leg rest extension; Overall length (fully reclined): 2,000 mm with telescopic backrest. |
| Precision | Stepwise positioning with 5 preset angles; Hydraulic dampening; Positional accuracy ±3°; Manual fine-tuning via foot control; Average transition time: 8 seconds (up/down). | Fully programmable memory with 8 user-defined positions; Servo-controlled incremental movement (0.5° resolution); Positional accuracy ±0.8°; Touchscreen + foot pedal + remote control interface; Transition time: 4.5 seconds (up/down), 6 seconds (full recline). |
| Material | Frame: Powder-coated carbon steel; Upholstery: Medical-grade polyurethane (PU) with antimicrobial coating; Armrests: Molded ABS plastic; Base: Cast aluminum with non-slip rubber pads. | Frame: Aerospace-grade aluminum alloy with anti-corrosion treatment; Upholstery: Seamless silicone-coated fabric, fluid-resistant and hypoallergenic; Armrests: Carbon fiber-reinforced polymer with adjustable height; Base: Stainless steel-reinforced composite with magnetic brake system. |
| Certification | CE Marked (Medical Device Directive 93/42/EEC); ISO 13485:2016 compliant; IEC 60601-1 (3rd Edition) safety standard; RoHS and REACH compliant. | CE Marked (MDR 2017/745); FDA 510(k) cleared; ISO 13485:2016 and ISO 14971:2019 (risk management); IEC 60601-1-2 (4th Edition) EMI/EMC; UL/CSA 60601-1 certified; Full traceability with UDI support. |
Note: All specifications are subject to change based on regional regulatory requirements and customization options. Advanced models support integration with digital clinic management systems (DICOM, HL7) and IoT-based predictive maintenance.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Chair Positions from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Release Date: Q1 2026
Executive Summary: China remains the dominant global manufacturing hub for dental chairs, representing 68% of worldwide exports (2025 WHO Medical Device Report). This guide details critical technical and commercial protocols for sourcing clinically validated dental chair positioning systems – the ergonomic core of modern dental units. Focus on positioning mechanisms (hydraulic/electric actuators, load capacity, range of motion) is essential for clinical safety and workflow efficiency.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Compliance validation is non-negotiable. Superficial certificate checks expose clinics to regulatory penalties and patient safety risks. Implement this technical verification protocol:
| Verification Level | Technical Requirements | Risk of Non-Compliance | Action Protocol |
|---|---|---|---|
| Documentary | Valid ISO 13485:2016 certificate (specific to dental chairs), CE Certificate of Conformity (MDD 93/42/EEC or MDR 2017/745), FDA 510(k) if targeting US distributors | Product seizure at customs; voided clinic insurance | Request certificate numbers; verify via EU NANDO database and ISO CertSearch |
| Technical File | EC Declaration of Conformity with full GSPR compliance, Risk Management File (ISO 14971), Clinical Evaluation Report (CER) | Recall liability; inability to register device in target market | Require redacted technical file excerpts covering positioning mechanism safety (IEC 60601-1, IEC 60601-2-37) |
| On-Site Audit | Witness load testing (min. 200kg dynamic load), positional accuracy validation (±1mm tolerance), emergency descent verification | Chronic chair failures; patient injury lawsuits | Engage 3rd party auditor (e.g., SGS, TÜV) for factory assessment; prioritize suppliers with in-house testing labs |
Step 2: Negotiating MOQ – Balancing Volume & Flexibility
Traditional Chinese suppliers enforce rigid MOQs, but market evolution enables strategic flexibility. Target these technical-commercial terms:
| Buyer Type | Standard MOQ (2026) | Negotiation Levers | Technical Compromise Assessment |
|---|---|---|---|
| Dental Clinics (Direct) | 5-10 units | Commit to multi-year service agreement; bundle with consumables (e.g., chair covers, lubricants) | Accept standard upholstery colors; minor positioning range reduction (max 5°) |
| Distributors (Regional) | 15-20 units | Pre-pay 30% for 15% MOQ reduction; co-branding for exclusive positioning features (e.g., pediatric mode) | Custom control panel firmware acceptable; hydraulic system standardization required |
| Distributors (National) | 30+ units | OEM/ODM commitment; inventory financing options; shared certification costs for new markets | Full customization (load capacity, positional memory slots, chair width) permitted |
Critical Note: Avoid suppliers requiring >30 units for standard chairs. This indicates outdated production capacity or inventory overhang.
Step 3: Shipping Terms – DDP vs. FOB Cost Analysis
Shipping terms directly impact landed costs and supply chain control. Technical components require specialized handling:
| Term | Cost Structure (Per Chair) | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Factory price: $1,850 • Ocean freight: $220 • Insurance: $45 • Destination charges: $185+ Total Est.: $2,290+ |
Buyer bears all cargo risk post-loading; customs clearance complexity; unexpected port demurrage | Experienced distributors with in-house logistics teams; shipments >20 HQ |
| DDP [Your Clinic] | • All-inclusive price: $2,350-$2,550 (Includes freight, insurance, duties, taxes, last-mile delivery) Total Est.: $2,450 (avg.) |
Supplier manages all risks; guaranteed delivery timeline; no hidden fees | 95% of clinics; first-time importers; urgent replacement needs; shipments <10 units |
Technical Shipping Requirement: Positioning mechanisms require climate-controlled containers (15-25°C) and anti-static packaging. Verify supplier’s IATA-certified medical device shipping protocols.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why They Excel in Dental Chair Positioning Systems (2026 Verified):
- Compliance Depth: ISO 13485:2016 + MDR 2017/745 certified with in-house testing lab (EMC, mechanical stress, biocompatibility)
- MOQ Flexibility: 3 units for clinics (DDP); 8 units for distributors with white-label options. No compromise on positioning mechanism specs.
- DDP Specialization: 35-day door-to-door delivery to 87 countries with climate-controlled logistics
- Technical Edge: Patented dual-motor positioning (0.1mm precision), 220kg load capacity, 24-month warranty on actuators
Factory Direct Engagement:
Shanghai Carejoy Medical Co., LTD | 19 Years OEM/ODM Expertise
Baoshan District, Shanghai, China (Dedicated Dental Equipment Industrial Park)
📧 [email protected] | 📱 WhatsApp: +86 15951276160
Request 2026 Technical Dossier: “DCP-2026 Positioning System Validation Report”
Implementation Roadmap
- Q1 2026: Shortlist 3 suppliers with verified ISO 13485 certificates (use NANDO/ISO databases)
- Q2 2026: Conduct virtual factory audit focusing on positioning mechanism assembly line
- Q3 2026: Negotiate DDP terms with sample validation (test positional repeatability 1,000 cycles)
- Q4 2026: Implement phased rollout with service-level agreement (SLA) for actuator repairs
Disclaimer: This guide reflects 2026 regulatory standards. Verify all specifications against local medical device regulations. Shanghai Carejoy is presented as a benchmark-compliant manufacturer based on 2025 third-party audit data (TÜV SÜD Report #CN2025-MED8873).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Equipment Distributors
Frequently Asked Questions: Dental Chair Positions for Patients (2026 Edition)
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing dental chair positions in 2026? | Modern dental chair systems in 2026 are designed for global compatibility, typically supporting dual-voltage inputs (100–240V, 50/60 Hz). However, ensure your clinic’s electrical infrastructure matches the chair’s specifications. Chairs with integrated digital controls, touchscreen interfaces, or IoT connectivity may require stable power with surge protection. Always verify local regulatory compliance (e.g., CE, FDA, ISO 60601-1) and consult the manufacturer’s technical datasheet prior to installation. |
| 2. Are spare parts for dental chair positions readily available, and what is the expected lead time? | Reputable manufacturers now offer comprehensive spare parts programs with guaranteed availability for a minimum of 10 years post-discontinuation. In 2026, modular design standards ensure faster replacement of components such as backrests, headrests, armrests, and hydraulic/pneumatic units. Lead times for in-stock parts average 3–7 business days globally, with expedited shipping options. Distributors are advised to maintain a strategic inventory of high-wear components (e.g., upholstery kits, foot control units, and tubing) to minimize chair downtime. |
| 3. What does the installation process for a new dental chair position involve, and is professional assistance required? | Installation of dental chair positions in 2026 requires certified biomedical technicians due to integrated digital systems, network connectivity, and utility connections (electric, water, air, and suction). The process includes site assessment, utility routing, leveling, system calibration, and software integration with clinic management platforms. Most manufacturers provide turnkey installation services within 48 hours of delivery. Pre-installation site audits are mandatory to ensure compliance with spatial, flooring, and electrical specifications. |
| 4. What warranty coverage is standard for dental chair positions in 2026, and what does it include? | Standard warranty terms in 2026 include a 3-year comprehensive coverage on mechanical, electrical, and electronic components, with extended warranty options up to 5 years. The warranty typically covers parts, labor, and on-site service for defects in materials or workmanship. Exclusions include damage from improper use, unauthorized modifications, or lack of preventive maintenance. Chairs with AI-assisted positioning or smart diagnostics may require annual service contracts to maintain full warranty eligibility. |
| 5. How are spare parts and warranty services managed for multi-location dental groups or large distributors? | Leading OEMs now offer centralized service portals for enterprise clients, enabling real-time tracking of spare parts inventory, warranty status, and service tickets across multiple clinics. Distributors benefit from dedicated technical support teams, bulk spare parts pricing, and co-branded service agreements. In 2026, predictive maintenance analytics integrated into chair systems allow proactive part replacement, reducing emergency service calls and optimizing equipment uptime for large-scale deployments. |
Need a Quote for Dental Chair Positions For Patient?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


