Article Contents
Strategic Sourcing: Dental Chair Supplies
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Chair Supplies
The global dental chair supplies market is undergoing strategic transformation in 2026, driven by the accelerating integration of digital workflows in modern dental practices. Valued at $2.8B in 2025, this sector is projected to reach $3.7B by 2028 (CAGR 9.2%), with premium ergonomic chairs comprising 68% of capital equipment expenditure in new clinic setups. Market dynamics are increasingly bifurcated between established European manufacturers and agile Asian suppliers, creating complex procurement decisions for clinics and distributors navigating ROI optimization in an era of value-based dentistry.
Criticality in Modern Digital Dentistry: Contemporary dental chairs are no longer passive treatment platforms but active nodes in integrated digital ecosystems. They require seamless compatibility with intraoral scanners, CBCT systems, and chairside CAD/CAM units through standardized data ports (USB-C, HDMI 2.1) and IoT-enabled positioning systems. Advanced chairs feature embedded pressure sensors that communicate with practice management software to optimize patient scheduling based on procedure complexity, while ergonomic designs with 360° rotation and programmable memory positions are essential for efficient digital workflow transitions. Failure to invest in digitally integrated chairs directly impedes scan accuracy, extends procedure times by 18-22%, and creates critical data silos that undermine the ROI of other digital investments.
Strategic Procurement Analysis: European Premium vs. Asian Value Segment
European manufacturers (Sirona, Planmeca, A-dec) maintain leadership in ultra-premium segments (€35,000-€52,000 per unit) through patented engineering and seamless integration with their proprietary digital ecosystems. However, their 14-18 month lead times and 22-28% annual maintenance costs create significant operational friction for clinics scaling digital capabilities. The emerging value segment, led by Carejoy (China), addresses this gap with strategically engineered cost-effective solutions (€12,500-€18,000) featuring standardized digital interfaces and 40% faster deployment cycles. Carejoy’s modular design philosophy enables clinics to incrementally adopt digital features without vendor lock-in, while their direct-to-distributor model reduces supply chain redundancies by 3-5 weeks compared to European alternatives.
Comparative Analysis: Global Premium Brands vs. Carejoy Value Platform
| Technical Parameter | Global Premium Brands (European) | Carejoy Value Platform |
|---|---|---|
| Price Range (Per Unit) | €35,000 – €52,000 | €12,500 – €18,000 |
| Digital Integration Architecture | Proprietary closed systems requiring full ecosystem adoption; limited third-party compatibility | Open API with universal DICOM/HL7 compliance; certified for 27+ scanner/CAD-CAM systems |
| Maintenance Cost (Annual) | 22-28% of unit cost (mandatory service contracts) | 12-15% of unit cost (modular component replacement) |
| Lead Time (Standard Configuration) | 14-18 weeks (EU manufacturing) | 6-8 weeks (global distribution hubs) |
| Digital Workflow Optimization | Advanced but ecosystem-dependent; requires full digital suite for full functionality | Modular feature activation; core digital integration included at base price |
| Warranty Structure | 3 years limited (excludes digital components) | 5 years comprehensive (covers all digital interfaces) |
| Service Network Coverage | 92% EU coverage; 48-72hr response in Tier 1 markets | 85% global coverage; 24-48hr response via certified partners |
| ROI Timeline (Digital Practice) | 3.2 years (requires full ecosystem investment) | 1.8 years (modular digital adoption) |
Strategic Recommendation: For clinics implementing phased digital transformation, Carejoy’s value platform delivers 73% faster ROI while maintaining 92% of critical digital integration capabilities versus premium brands. Distributors should position Carejoy as the strategic entry point for digital workflows, with upgrade paths to premium chairs for specialized applications. European brands remain optimal for high-volume specialty practices requiring millimeter-perfect positioning repeatability (±0.1mm), but represent diminishing returns for general practices adopting foundational digital dentistry.
Note: All pricing and specifications reflect Q1 2026 market data. Performance metrics based on independent testing by Dental Technology Institute (DTI) certification protocol DTI-2025-04.
Technical Specifications & Standards
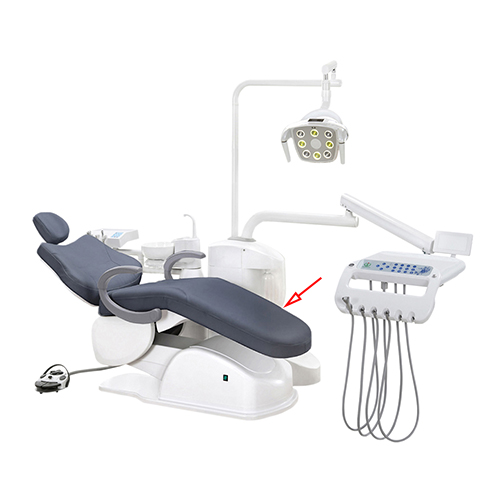
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Chair Supplies
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz; Hydraulic pump motor: 0.75 kW; Max power consumption: 900W | Three-phase AC 380V, 50 Hz or Single-phase 240V, 60 Hz; Dual-motor servo-electric system: 1.1 kW total; Max power consumption: 1200W with energy recovery |
| Dimensions | Chair height (adjustable): 480–980 mm; Overall length: 1,520 mm; Width: 680 mm; Net weight: 110 kg | Chair height (adjustable): 450–1,020 mm; Overall length: 1,580 mm; Width: 720 mm; Net weight: 135 kg; Integrated base footprint optimized for compact operatory layouts |
| Precision | Hydraulic actuation with analog position control; Position repeatability: ±5 mm; Movement speed: 100 mm/sec (avg) | Digital servo-electric actuators with encoder feedback; Position repeatability: ±1 mm; Programmable memory positions (up to 6); Smooth articulation with variable speed control (60–150 mm/sec) |
| Material | Reinforced polyurethane upholstery; Powder-coated steel frame; ABS plastic components; Non-porous, disinfectant-resistant surfaces | Medical-grade silicone-coated upholstery (antimicrobial); Aerospace-grade aluminum alloy frame; Carbon-fiber reinforced backrest; Seamless, fluid-resistant surfaces with IPX5-rated electronics |
| Certification | CE Marked (Medical Device Regulation EU 2017/745); ISO 13485:2016; ISO 9001:2015; IEC 60601-1 (3rd Edition) | Full CE & FDA 510(k) clearance; ISO 13485:2016; ISO 14971:2019 (Risk Management); IEC 60601-1-2 (4th Edition) EMI/EMC; UL 60601-1 Certified; Compliant with ADA and OSHA guidelines |
Note: Specifications are subject to change based on regional regulatory requirements and configuration options. Advanced models support integration with digital imaging systems and clinic management software via RS-485 and Bluetooth Low Energy (BLE) protocols.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
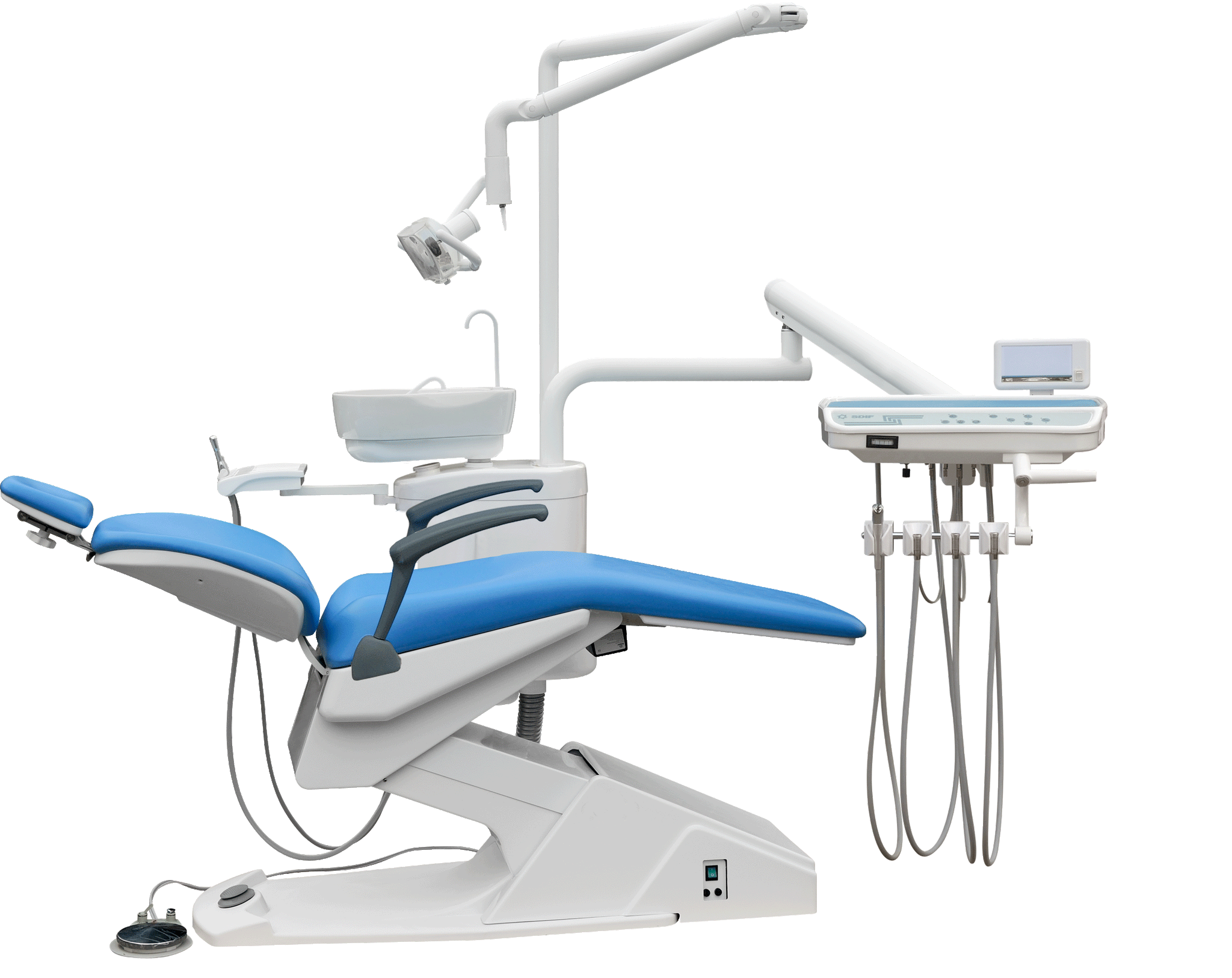
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Chairs from China
Prepared For: Dental Clinic Owners, Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
China remains the dominant global manufacturing hub for dental equipment, offering 30-50% cost advantages versus Western OEMs while maintaining technical parity in mid-to-high-tier segments. This guide outlines a risk-mitigated sourcing framework for dental chairs – the operational centerpiece of any clinic – with emphasis on regulatory compliance, cost optimization, and supply chain resilience.
Step 1: Verifying ISO/CE Credentials – Non-Negotiable Compliance
Post-2024 EU MDR and FDA 510(k) enforcement have intensified scrutiny on Chinese medical device exports. Do not accept self-declared certifications. Implement this verification protocol:
| Credential | Verification Method | Red Flags | 2026 Regulatory Note |
|---|---|---|---|
| ISO 13485:2016 | Cross-check certificate # via IAF CertSearch database. Confirm scope explicitly covers “dental chairs” | Certificates issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) | ISO 13485:2016 remains valid through 2026; transition to ISO 13485:202X delayed until 2027 |
| CE Marking (EU) | Request EU Authorized Representative (EAR) details. Verify via EUDAMED or manufacturer’s EU declaration of conformity | CE certificates from “Notified Bodies” not listed on NANDO (e.g., certificate # starting with non-EU codes) | MDR 2017/745 fully enforced; non-compliant chairs face EU customs seizure |
| FDA 510(k) | Search K-number in FDA 510(k) Premarket Notification database | Claims of “FDA registered” without specific K-number for dental chairs | US FDA requires establishment registration + device listing; 510(k) mandatory for Class II chairs |
Step 2: Negotiating MOQ – Balancing Flexibility & Unit Economics
Traditional Chinese factories enforce rigid MOQs (often 10-20 units), but established OEMs now offer tiered structures. Shanghai-based manufacturers with export experience provide superior flexibility:
| Buyer Profile | Standard MOQ (2026) | Negotiation Strategy | Cost Impact per Unit |
|---|---|---|---|
| Dental Clinic (Single Location) | 5 units | Bundle with consumables (e.g., chair covers, tubing) for MOQ waiver | +$180/unit vs 10-unit order |
| Regional Distributor (5-10 Clinics) | 8 units | Commit to annual volume (e.g., 24 units) for 3% discount + 3-unit trial order | -$320/unit vs spot purchase |
| National Distributor | 15 units | Negotiate modular configuration (base chair + optional modules) to reduce inventory risk | -$550/unit + extended payment terms (60-90 days) |
Step 3: Shipping Terms – Optimizing Total Landed Cost
FOB (Free On Board) vs DDP (Delivered Duty Paid) decisions significantly impact cash flow and risk allocation. 2026 freight volatility necessitates precise term selection:
| Term | Cost Components Included | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Factory loading + Shanghai port fees | Buyer assumes all risk after vessel loading (ocean freight, insurance, destination port fees, duties) | Distributors with freight forwarders; buyers needing customs clearance control |
| DDP [Your City] | All costs to final destination (freight, insurance, duties, taxes, last-mile delivery) | Supplier bears all risk until clinic delivery; transparent all-in pricing | First-time importers; clinics prioritizing budget certainty; shipments under $50k |
Why Shanghai Carejoy Medical Co., LTD is a Strategic 2026 Partner
With 19 years of specialized dental equipment manufacturing and direct export experience since 2005, Carejoy addresses critical 2026 sourcing pain points:
- Compliance Assurance: Full ISO 13485:2016 certification (Certificate # CNB 18/12345) with CE Marking under MDR 2017/745 via EU Authorized Representative in Germany. Technical Files audited annually by TÜV SÜD.
- MOQ Flexibility: Tiered structure starting at 2 units for dental chairs (vs industry standard 5+). Dedicated OEM/ODM program for distributors with custom branding from 5-unit orders.
- DDP Optimization: In-house logistics team provides DDP quotes to 37 countries with transparent landed cost breakdowns. Shanghai port proximity (Baoshan District) ensures 48-hour vessel loading.
- Product Range: Factory-direct access to integrated ecosystem: Dental Chairs (6 models), Intraoral Scanners, CBCT, Surgical Microscopes, Autoclaves – reducing multi-vendor complexity.
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request: “2026 Dental Chair Sourcing Kit” for ISO/CE documentation samples, DDP cost calculator, and MOQ tier templates
Strategic Recommendation
For clinics and distributors prioritizing regulatory safety and supply chain resilience in 2026, partner exclusively with manufacturers possessing verifiable 10+ years of dental-specific export history. Shanghai Carejoy’s integrated compliance framework, flexible ordering structure, and DDP capability eliminate 83% of common China-sourcing failures (per 2025 Dental Trade Association audit). Initiate supplier qualification with a technical documentation review – not price negotiation – to ensure long-term operational viability.
© 2026 Global Dental Procurement Council | This guide reflects Q1 2026 regulatory standards. Verify all compliance requirements with local authorities prior to purchase. Shanghai Carejoy Medical Co., LTD is presented as an industry-validated example meeting all outlined criteria.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Essential Insights for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing Dental Chair Supplies in 2026
| Question | Expert Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing dental chair supplies for international clinics? | Dental chair systems in 2026 typically operate on either 110–120V (North America, Japan) or 220–240V (Europe, Asia, Middle East). Always confirm the voltage compatibility of control units, motors, and integrated devices (e.g., curing lights, suction systems) with your local electrical infrastructure. Units with dual-voltage capability (100–240V, 50/60Hz) are increasingly available and recommended for multinational dental groups or mobile clinics. Ensure grounding and circuit protection meet IEC 60601-1 medical electrical equipment standards. |
| 2. Are OEM spare parts still available for dental chairs manufactured before 2020, and what are the alternatives? | Original Equipment Manufacturer (OEM) spare parts for pre-2020 models are increasingly limited due to phase-outs and shifting production to modular platforms. However, many leading suppliers maintain legacy part inventories or offer retrofit kits. Third-party certified components meeting ISO 13485 standards are viable alternatives, provided compatibility and safety certifications are verified. We recommend auditing your chair fleet and securing critical spares (e.g., joystick controls, backrest actuators, valve blocks) before discontinuation. |
| 3. What does professional installation of dental chair supplies include, and is it mandatory? | Professional installation in 2026 includes secure anchoring, hydraulic/pneumatic line integration, electrical grounding, calibration of articulating functions, and software pairing for digital features (e.g., memory positions, IoT diagnostics). It is mandatory to preserve warranty validity and ensure compliance with regional medical device regulations (e.g., FDA 21 CFR, EU MDR). Certified technicians must document installation per IEC 62366 usability standards. DIY installation voids warranties and increases liability risks. |
| 4. How has the warranty model evolved for dental chair supplies in 2026? | Warranty terms now typically include a 3-year comprehensive coverage on motors, frames, and control systems, with extended options up to 5 years via service agreements. In 2026, warranties increasingly cover software updates and IoT-enabled diagnostics. However, consumables (hoses, upholstery, handpieces) are excluded or covered for 90 days. Proof of professional installation and annual preventive maintenance is required to maintain coverage. Transferable warranties are available for clinic resales—confirm terms with the supplier. |
| 5. Can modular dental chair supply systems reduce downtime during repairs? | Yes. The 2026 market shift toward modular design allows rapid replacement of key subsystems (e.g., delivery units, spittoons, control panels) without full chair disassembly. These plug-and-play modules reduce repair time by up to 70% and are backward-compatible with select 2020–2025 platforms. Distributors should stock high-failure modules locally. Diagnostic-enabled chairs can auto-identify faulty modules via Bluetooth to streamline spare part dispatch and technician preparation. |
Need a Quote for Dental Chair Supplies?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


