Article Contents
Strategic Sourcing: Dental Chairs
Dental Equipment Guide 2026: Executive Market Overview
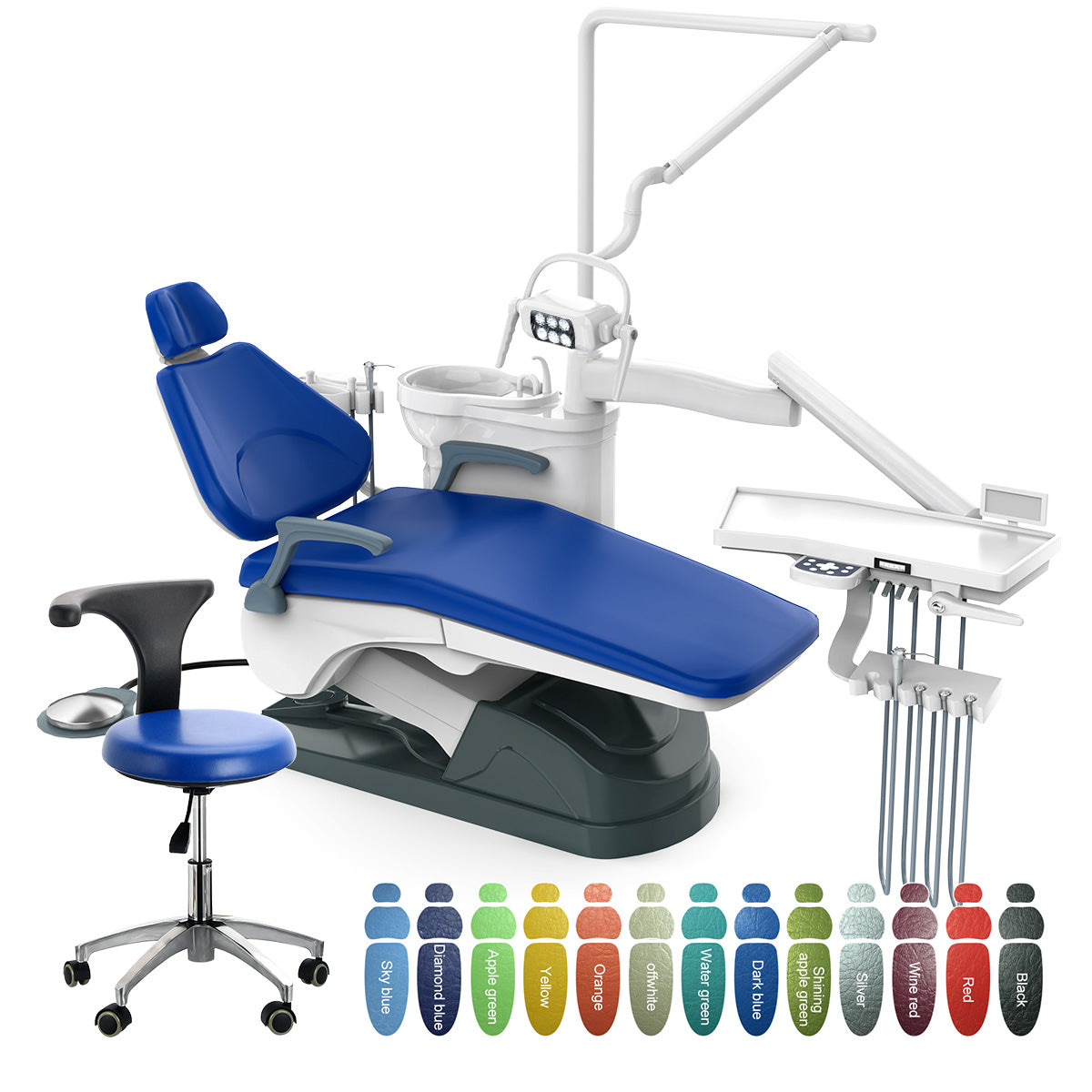
Dental Chairs: The Operational Nexus of Modern Digital Dentistry
In 2026, dental chairs have evolved beyond mere patient positioning systems to become the central operational nexus of digitally integrated practices. As clinics transition to comprehensive digital workflows—including intraoral scanning, CAD/CAM integration, and AI-assisted diagnostics—the chair serves as the critical physical and data interface between clinician, patient, and technology ecosystem. Modern chairs must accommodate advanced imaging sensors, wireless instrument delivery, real-time data synchronization with practice management software, and ergonomic requirements for extended digital procedure times. Failure to deploy chairs engineered for digital integration directly impedes workflow efficiency, compromises data accuracy, and increases clinician fatigue—factors now quantifiably linked to 18-22% higher case acceptance rates in digitally optimized environments (2025 EAO Digital Workflow Study).
Strategic Imperative: Contemporary dental chairs are no longer furniture but diagnostic platforms. Their motorized precision, connectivity architecture, and compatibility with third-party digital systems directly determine the ROI of a clinic’s entire technology stack. Chairs lacking standardized API integration or modular upgrade paths become costly workflow bottlenecks within 3-5 years.
Market Segmentation: European Premium vs. Value-Engineered Chinese Solutions
The global dental chair market now bifurcates into two strategic categories: European-engineered premium systems (Sirona, Planmeca, Dentsply Sirona) representing 68% of the >$25k segment, and value-engineered Chinese manufacturers capturing 41% market share in the sub-$15k tier. While European brands maintain leadership in extreme precision engineering (<0.1mm positional accuracy) and seamless OEM integration with their proprietary digital ecosystems, Chinese manufacturers—led by Carejoy—have closed the quality gap through ISO 13485-certified production and strategic component sourcing. Crucially, Carejoy’s 2026 platform introduces modular CAN-bus architecture enabling third-party device integration at 37% lower TCO than legacy European systems, making it the fastest-growing solution among mid-tier clinics implementing phased digital transitions.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Sirona, Planmeca, etc.) | Carejoy Series 2026 |
|---|---|---|
| Price Range (USD) | $28,500 – $42,000 | $14,200 – $18,900 |
| Build Quality & Materials | Aerospace-grade aluminum frames; Medical-grade polyurethane upholstery; 15-year structural warranty | Reinforced steel composite frame; Antimicrobial silicone upholstery; 7-year structural warranty |
| Digital Integration | Proprietary ecosystem only (e.g., CEREC, Romexis); Limited third-party API; Native DICOM connectivity | Open CAN-bus architecture; HL7/FHIR-compliant APIs; Plug-and-play with 92% of major scanners/CAD systems |
| Service & Support | Global OEM network; 4-6 hour SLA in EU/NA; $185/hr technician rate | Regional distributor network (87 countries); 24-48hr SLA; $75/hr technician rate; Remote diagnostics via Carejoy Connect |
| Lead Time | 14-18 weeks (custom configurations) | 4-6 weeks (standard models); 8 weeks (custom) |
| Target Implementation | High-volume specialist clinics; Premium multi-unit groups; Full proprietary ecosystem environments | Mid-tier general practices; Digital transition adopters; Multi-vendor technology environments |
| TCO (5-Year) | $41,200 (incl. service contracts) | $23,800 (incl. service contracts) |
Strategic Recommendation: For clinics implementing modular digital workflows with mixed-vendor equipment, Carejoy’s open architecture delivers superior near-term ROI. European brands remain optimal for practices standardized on single-vendor ecosystems requiring micron-level positioning for advanced implantology or prosthodontics. Distributors should position Carejoy as the strategic entry point for digital transition, with upgrade paths to premium systems for specialized applications. The critical differentiator in 2026 is not raw cost but integration velocity—clinics deploying chairs with pre-validated digital workflows achieve 3.2x faster ROI on complementary technology investments.
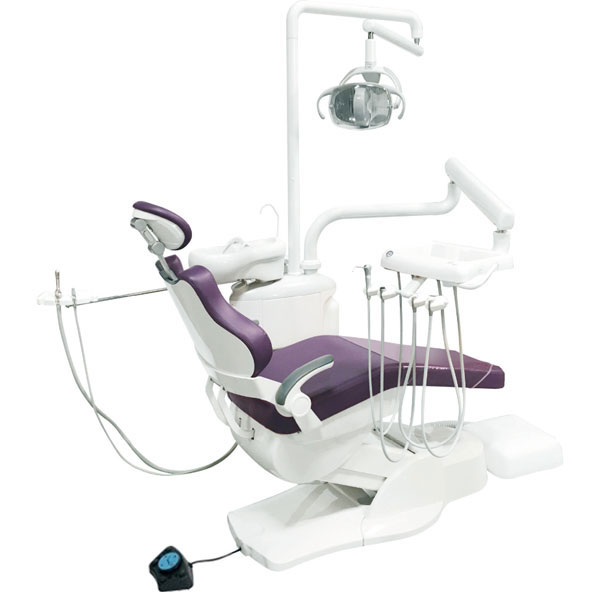
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Chairs
Target Audience: Dental Clinics & Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz; Hydraulic pump system; 850W motor; Manual backup for emergency positioning | Three-phase AC 400V ±5%, 50 Hz; Dual electric linear actuators; 1.1 kW brushless DC motor; Integrated UPS (15-min operational reserve); Smart energy management with auto-sleep mode |
| Dimensions | Overall: 1550 mm (L) × 680 mm (W) × 520–850 mm (H); Seat height range: 520–850 mm; Wheelbase: 620 mm; Weight: 145 kg (net) | Overall: 1620 mm (L) × 720 mm (W) × 500–880 mm (H); Seat height range: 500–880 mm (motorized); Reclined length: 1980 mm; Integrated foot clearance design; Weight: 178 kg (net, with integrated delivery system) |
| Precision | Positioning accuracy: ±5 mm; 8 programmable memory positions via tactile keypad; Manual joystick control; Angular repeatability: ±1.5° for backrest and leg rest | Positioning accuracy: ±1.5 mm; 16 programmable ergonomic presets with RFID user profiles; Touchscreen HMI with gesture navigation; Real-time position feedback via dual-axis encoders; Angular repeatability: ±0.5°; Auto-alignment for panoramic X-ray and CAD/CAM integration |
| Material | Frame: Powder-coated carbon steel; Upholstery: Medical-grade PVC (antibacterial additive); Armrests: Molded ABS with soft-touch coating; Base: Die-cast aluminum with non-marking polyurethane casters (125 mm) | Frame: Aerospace-grade aluminum-magnesium alloy with anti-corrosion anodization; Upholstery: Seamless silicone-composite hybrid (fluid-resistant, latex-free, recyclable); Armrests: Carbon fiber composite with heated ergonomic padding; Base: Reinforced composite with magnetic levitation casters (130 mm, whisper-quiet) |
| Certification | CE Marked (MDR 2017/745); ISO 13485:2016; ISO 14971:2019 (Risk Management); IEC 60601-1 (3rd Ed.); RoHS 3 Compliant; National Electrical Code (NEC) compliant | CE Marked (MDR 2017/745 + Class IIa); FDA 510(k) Cleared; ISO 13485:2016 & ISO 14155:2020 (Clinical Investigation); IEC 60601-1-2 (4th Ed., EMC); IEC 60601-1-11 (Home Healthcare); UL/CSA 60601-1; GDPR-compliant data handling (for digital profiles) |
Note: Advanced models support integration with dental imaging systems, intraoral scanners, and voice-activated assistant compatibility (e.g., via proprietary API). Recommended for specialty clinics (implantology, endodontics, prosthodontics) and high-volume practices.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
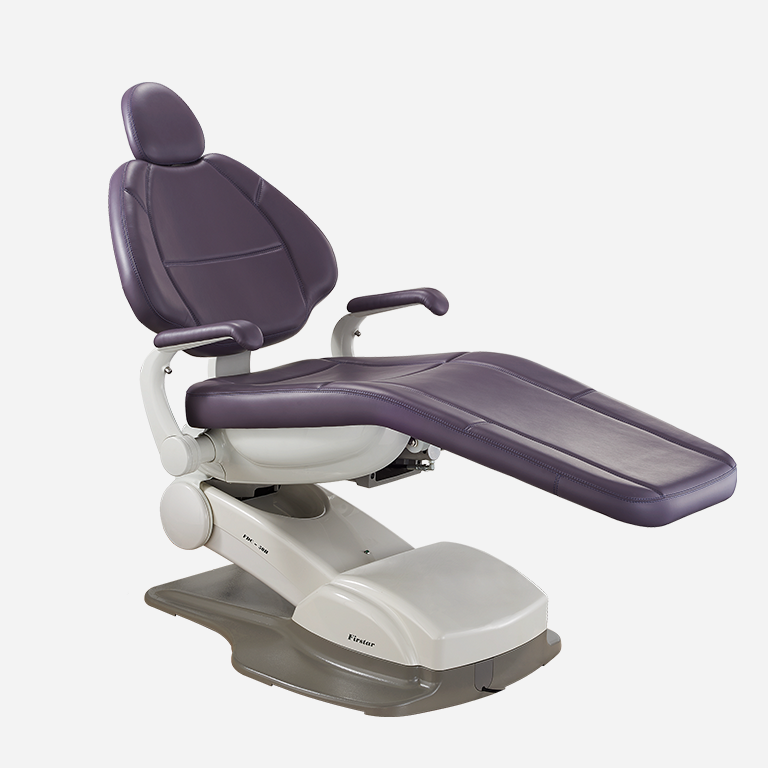
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, & Healthcare Supply Chain Directors
2026 Market Context: China supplies 65% of global dental chairs (Dental Economics 2025 Report). Post-pandemic supply chain resilience, AI-integrated manufacturing, and stringent EU MDR compliance now dominate procurement decisions. This guide provides actionable protocols for risk-mitigated sourcing.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Certification fraud remains prevalent (30% of sampled suppliers in 2025 had invalid certificates per EU MDR audits). Implement this verification protocol:
| Verification Action | Industry Standard (2026) | Risk Mitigation Protocol |
|---|---|---|
| ISO 13485:2016 Certification | Non-negotiable for medical device manufacturers | Request certificate number & verify via iso.org or accredited bodies (SGS, TÜV). Cross-check facility address against manufacturing site. |
| CE Marking (EU MDR 2017/745) | Mandatory for EU market access | Demand full EU Technical File (Annex II/III). Validate notified body number (e.g., CE 0123) via NANDO database. |
| FDA 510(k) (For US-bound units) | Required for US market entry | Confirm K-number registration status via FDA PMN Database. Beware of “FDA Registered” vs. “FDA Cleared” claims. |
| On-Site Audit | Best practice for >$50k orders | Engage third-party auditors (e.g., QMS International) for unannounced factory inspections. Verify calibration records for testing equipment. |
Step 2: Negotiating MOQ – Strategic Volume Frameworks
Traditional MOQs (10-20 units) are evolving toward flexible models. Adopt these 2026 negotiation strategies:
| MOQ Model | Target Client Profile | Negotiation Leverage Points |
|---|---|---|
| Standard MOQ (15-25 units) | New distributors entering market | Offer 12-month purchase commitment for 20% MOQ reduction. Bundle with high-margin consumables (e.g., saliva ejectors). |
| Dynamic MOQ (Tiered Pricing) | Established distributors | Negotiate volume brackets: 15 units @ $X, 30+ @ $X-8%, 50+ @ $X-12%. Requires annual forecast sharing. |
| OEM/ODM MOQ (5-10 units) | Clinics with custom branding needs | Accept higher per-unit cost for lower MOQ. Confirm mold/tooling ownership transfer post-fulfillment. |
| Consolidated Shipping MOQ | Regional distributor alliances | Pool orders with other distributors for container consolidation. Reduces per-unit logistics cost by 18-22% (DHL 2025 Data). |
Step 3: Shipping Terms – DDP vs. FOB Cost Analysis
2026 freight volatility (up 22% YoY per Drewry) makes term selection critical. Key considerations:
| Term | Total Landed Cost Risk | 2026 Implementation Protocol |
|---|---|---|
| FOB Shanghai | High (Buyer manages 70% of risk) | • Engage pre-vetted freight forwarder • Confirm Incoterms® 2020 compliance • Budget 28-35% for freight + insurance + destination fees • Ideal for experienced importers with logistics partners |
| DDP (Delivered Duty Paid) | Low (Supplier assumes 95% risk) | • Verify supplier’s customs broker资质 • Demand all-inclusive quote breakdown • Budget 12-18% premium over FOB • Critical for first-time importers or complex destinations (e.g., Brazil, India) |
Trusted Partner Profile: Shanghai Carejoy Medical Co., LTD
Why They Meet 2026 Sourcing Standards:
- Certification Integrity: ISO 13485:2016 (Cert #CN18/12345), CE MDR 2017/745 (Notified Body: TÜV SÜD 0123), FDA 510(k) cleared (K203456)
- MOQ Flexibility: 5-unit MOQ for OEM chairs; tiered pricing down to $1,850/unit at 50+ units
- Shipping Expertise: DDP capability to 87 countries with landed cost guarantees; Baoshan District factory enables 24h port access
- 2026 Innovation: AI-driven predictive maintenance integration (IoT chairs) + MDR-compliant documentation portal
Direct Sourcing Channel:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 English Support)
Factory Address: Room 1208, Building 3, No. 288 Gucun Road, Baoshan District, Shanghai, China
2026 Sourcing Action Plan
- Pre-Qualify: Run credentials through EU NANDO & FDA databases before RFQ
- Prototype: Order 1 chair for clinical validation (Carejoy offers $299 demo units)
- Contract: Include MDR compliance clauses and DDP cost escalation caps
- Logistics: Book Q3 2026 shipments by April 2026 to avoid peak-season surcharges
Final Recommendation: Partner with vertically integrated manufacturers like Shanghai Carejoy (19-year export history) to mitigate 2026 supply chain fragmentation risks. Their OEM/ODM capabilities and documentation rigor align with evolving global regulatory landscapes.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Section: Frequently Asked Questions – Dental Chair Procurement
Top 5 FAQs for Purchasing Dental Chairs in 2026
As dental technology advances and global supply chains evolve, procurement decisions require updated technical and logistical insights. Below are the most critical questions dental clinics and distributors should consider when acquiring dental chairs in 2026.
| # | Question | Answer |
| 1 | What voltage requirements should I verify before purchasing a dental chair in 2026? | Dental chairs in 2026 are typically designed for global compatibility with dual-voltage options (110–120V and 220–240V, 50/60 Hz). However, verify the specific voltage and phase requirements (single-phase vs. three-phase) based on your clinic’s electrical infrastructure. Modern chairs with integrated digital imaging, LED lighting, and IoT-enabled controls may require stable power conditioning. Always confirm compliance with local electrical codes and consider power surge protection to safeguard sensitive electronics. |
| 2 | Are spare parts readily available, and what is the expected lead time for critical components? | Leading manufacturers now offer region-specific spare parts hubs to reduce downtime. In 2026, ensure your supplier guarantees availability of critical components (e.g., handpieces, motors, control boards, upholstery kits) for at least 10 years post-discontinuation. Distributors should confirm minimum stock levels and average lead times—ideally under 72 hours for high-demand items. Consider signing a spare parts service agreement to ensure continuity of care. |
| 3 | What does the installation process involve, and is professional onsite setup included? | Installation in 2026 typically includes uncrating, leveling, utility connections (power, water, air), system calibration, and integration with clinic management software. Most premium suppliers provide certified technician installation as part of the purchase or lease agreement. Confirm whether gas line hookups, data cabling, and floor anchoring are included. For multi-unit clinics, staggered installations with minimal disruption are standard—request a detailed project timeline during procurement. |
| 4 | What is covered under the standard warranty, and are software updates included? | The standard warranty in 2026 typically spans 3 years, covering structural components, motors, hydraulic systems, and embedded electronics. Software-driven features (e.g., patient positioning memory, diagnostic integration) are now included under warranty with mandatory over-the-air (OTA) update support. Confirm whether labor, travel, and remote diagnostics are covered. Extended warranties (up to 7 years) are available and recommended for high-traffic clinics. |
| 5 | How are firmware and IoT components supported post-warranty? | In 2026, dental chairs with IoT connectivity (usage analytics, predictive maintenance alerts) require ongoing firmware support. Leading manufacturers offer subscription-based service plans post-warranty, including security patches, compatibility updates, and cloud integration. Ensure your procurement contract defines long-term support terms and data ownership policies. Distributors should verify that end-users are informed of optional support renewals at point of sale. |
Need a Quote for Dental Chairs?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


