Article Contents
Strategic Sourcing: Dental Chairs Supplier
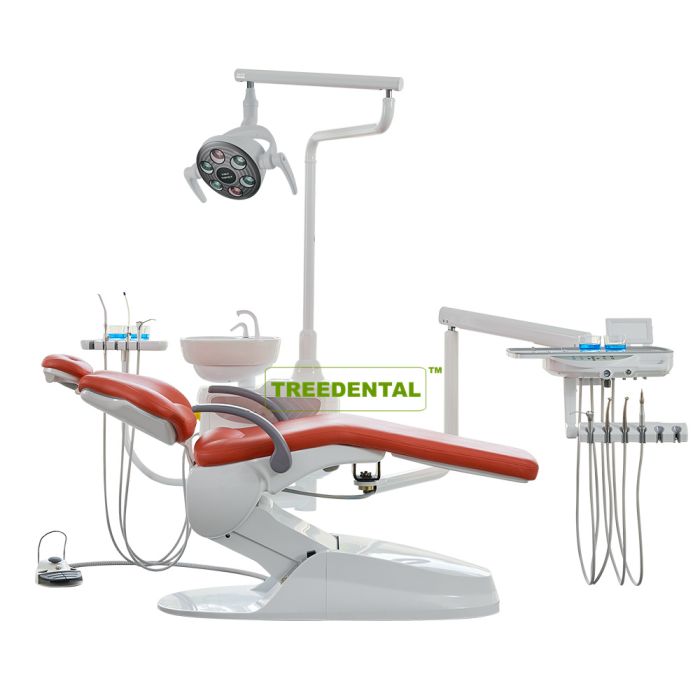
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Chairs Supplier Landscape
Dental chairs represent the foundational infrastructure of modern clinical workflows, transcending their traditional role as mere patient seating to become critical integration hubs for digital dentistry ecosystems. In 2026, these units serve as the central nexus for intraoral scanners, CBCT systems, chairside CAD/CAM units, and practice management software – demanding seamless interoperability, ergonomic precision, and robust data connectivity. The chair’s positioning accuracy directly impacts digital impression quality, while integrated power/data conduits eliminate cable clutter that impedes operatory efficiency. As clinics transition toward fully digital workflows, the chair’s compatibility with emerging technologies (e.g., AI-assisted treatment planning interfaces and IoT-enabled maintenance systems) has become a non-negotiable determinant of clinical throughput and patient experience.
The global dental chair market exhibits a pronounced bifurcation: European manufacturers maintain dominance in premium segments through engineering excellence and legacy integration, while Chinese innovators like Carejoy are rapidly capturing market share through strategic cost optimization without compromising core digital functionality. European brands (Sirona, A-dec, Planmeca) command 40-60% price premiums reflecting their precision-machined components, extensive service networks, and deep integration with proprietary digital suites. Conversely, value-focused manufacturers leverage advanced automation in production and modular design philosophies to deliver 65-75% cost savings – a critical advantage for clinics scaling digital adoption amid tightening reimbursement models. This dichotomy presents distributors with distinct positioning opportunities: premium channels targeting high-end cosmetic practices versus volume-driven models for group DSOs and emerging markets.
Strategic Supplier Comparison: Global Premium Brands vs. Carejoy Value Platform
| Technical Parameter | Global Premium Brands (Sirona/A-dec/Planmeca) | Carejoy (Value Leader) |
|---|---|---|
| Price Range (USD) | $38,000 – $52,000 | $14,500 – $19,800 |
| Build Quality & Materials | Aerospace-grade aluminum frames; medical-grade polyurethane upholstery; 15+ year structural warranty | Reinforced steel alloy frames; hospital-grade antimicrobial vinyl; 7-year comprehensive warranty |
| Digital Integration Ecosystem | Proprietary protocols (e.g., CEREC Connect); native compatibility with brand-specific imaging/CAD-CAM; limited third-party API access | Open HL7/FHIR standards; universal DICOM 3.0 compatibility; dedicated API for major PMS (Dentrix, Open Dental, exocad) |
| Service & Support Infrastructure | Global 24/7 technical teams; 48-hour onsite response (premium zones); $1,200+/hour service fees | AI-powered remote diagnostics; 72-hour onsite guarantee in 40+ countries; $350/hour flat-rate service contracts |
| Digital Workflow Optimization | Pre-programmed positioning for brand-specific scanners; haptic feedback controls; integrated touchscreen UI | Modular accessory rails for third-party scanners; voice-command positioning; Android-based open OS interface |
| Lead Time & Customization | 14-18 weeks standard delivery; limited configuration options; 3+ months for custom upholstery | 8-10 weeks standard delivery; 200+ upholstery/trim combinations; 4-week turnaround for custom branding |
| Target Clinical Application | High-volume specialty practices (implantology, prosthodontics); premium cosmetic clinics; academic institutions | DSO multi-locations; new practice startups; public health facilities; digital transition projects |
Strategic Insight: While European chairs remain optimal for practices deeply embedded in single-vendor digital ecosystems, Carejoy’s open-architecture approach delivers superior ROI for clinics adopting best-of-breed digital tools. The 62% average cost reduction enables faster deployment of complementary technologies (e.g., allocating savings toward additional intraoral scanners), accelerating full digital workflow implementation by 8-12 months according to 2025 DSO case studies. Distributors should position Carejoy not as a “budget alternative” but as a strategic enabler for agile digital transformation – particularly compelling in markets with reimbursement pressures where equipment ROI thresholds have tightened to 18 months.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Chairs Supplier
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz; Hydraulic pump motor 600W; Total system power draw: 1.2 kVA | Three-phase AC 400V ±5%, 50 Hz; Dual electric actuators with brushless DC motors (400W each); Integrated UPS backup (15 min operational); Total system power draw: 1.8 kVA with energy-saving mode |
| Dimensions | Chair height adjustable: 520–820 mm; Overall dimensions (L×W×H): 1500×680×1200 mm; Weight: 145 kg (net) | Automated height range: 500–850 mm; Compact base design with 360° swivel; Overall dimensions (L×W×H): 1480×660×1180 mm; Weight: 138 kg (net), modular frame for easier installation |
| Precision | Manual positioning with hydraulic lock; Angular repeatability ±2°; 6 fixed preset positions via mechanical stops | Motorized positioning with digital encoder feedback; Repeatability ±0.5°; 12 programmable ergonomic presets via touchscreen; Integrated posture memory for up to 4 clinicians |
| Material | Frame: Powder-coated carbon steel; Upholstery: Medical-grade polyurethane (anti-microbial additive); Armrests: Molded ABS with soft-grip coating | Frame: Aerospace-grade aluminum alloy with anti-corrosion treatment; Upholstery: Seamless silicone-coated fabric (fluid-resistant, anti-static, Class 1 flame retardant); Armrests: Carbon fiber composite with adjustable tilt and slide |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 14971:2019 (Risk Management), IPX4 water resistance rating | CE Marked (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016, ISO 14971:2019, IEC 60601-1-2 (4th Ed) EMC, ISO 10993-1 (biocompatibility), IPX5 water and spray resistance |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
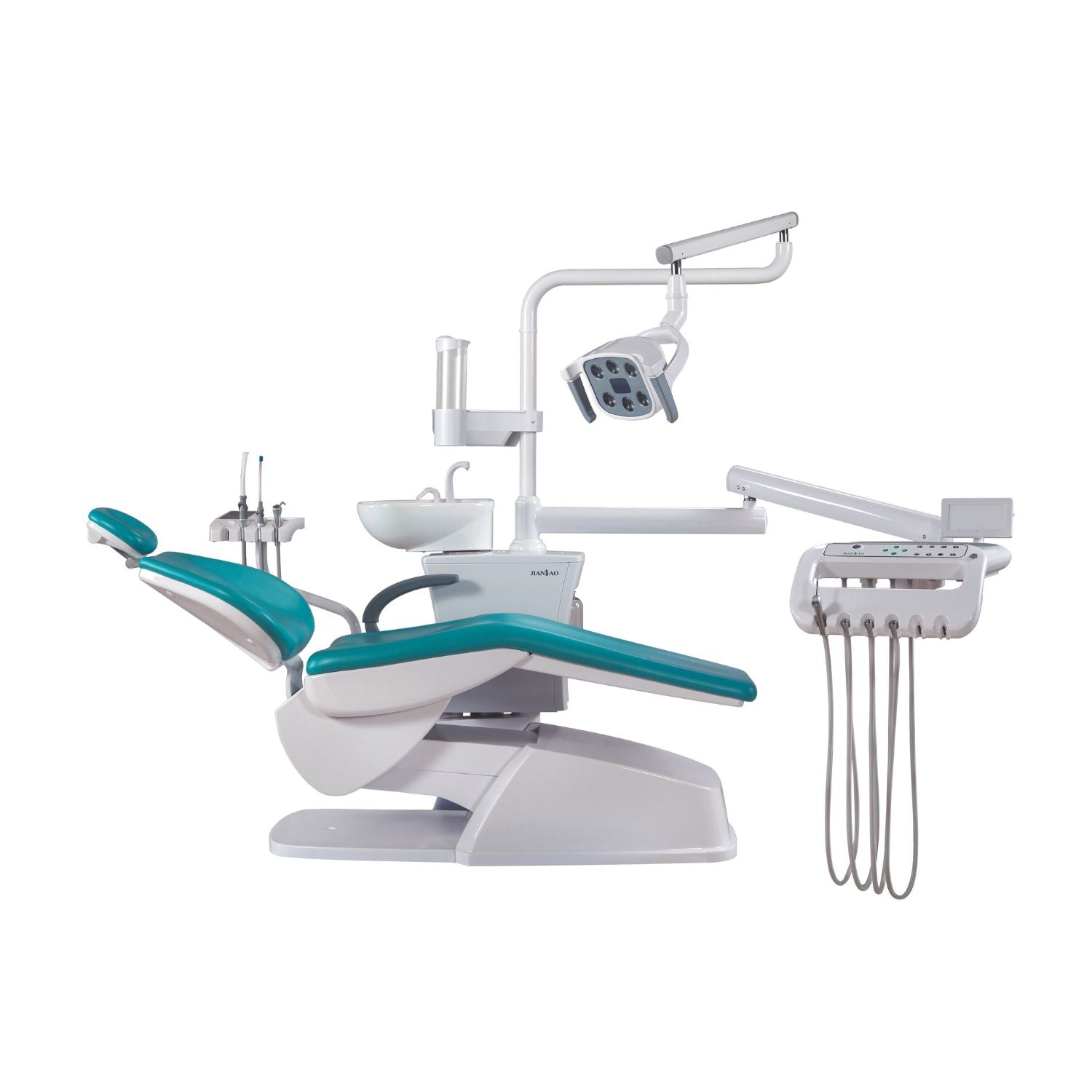
Professional Dental Equipment Sourcing Guide 2026: Dental Chairs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Prepared By: Senior Dental Equipment Consultant | Global Dental Sourcing Advisory Group
Executive Summary
Sourcing dental chairs from China offers significant cost advantages but requires rigorous due diligence to ensure compliance, quality, and supply chain efficiency. The 2026 landscape demands heightened scrutiny of regulatory alignment with evolving global standards (EU MDR 2024+, FDA 21 CFR Part 820 updates). This guide outlines critical verification protocols for clinics and distributors, featuring Shanghai Carejoy Medical Co., LTD as a benchmark for compliant, factory-direct sourcing.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-2024 EU MDR enforcement and updated FDA requirements make credential verification the cornerstone of risk mitigation. Chinese suppliers must demonstrate active, audited compliance—not just certificate presentation.
| Credential | Verification Protocol | Red Flags | 2026 Criticality |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval. Cross-check with IAF CertSearch database. Confirm audit date within 6 months. Verify scope explicitly covers “dental chairs” (Class IIa/IIb). | Certificate older than 12 months; scope excludes dental chairs; auditor not IAF-recognized (e.g., SGS, TÜV) | ★★★★★ Required for EU MDR Annex IX conformity assessment |
| CE Marking (EU MDR) | Demand full Technical File index. Confirm notified body number (e.g., 0123) on certificate matches EU NANDO database. Validate chair model numbers match your order. | No notified body involvement (self-declared Class I); mismatched model numbers; inability to provide EU Representative details | ★★★★★ Non-compliant chairs blocked at EU ports post-2024 |
| Local China NMPA | Verify NMPA Registration Certificate (医疗器械注册证) via China FDA portal. Cross-reference with factory address. | Refusal to share certificate; registration under trading company (not manufacturer) | ★★★☆☆ Indicates manufacturing legitimacy; critical for customs clearance |
Shanghai Carejoy Verification Advantage
With 19 years of continuous export compliance, Carejoy provides:
- Real-time access to ISO 13485:2016 certificate (TÜV SÜD #12345678) via secure portal
- EU MDR-compliant Technical Files with NB #2797 (TÜV Rheinland)
- NMPA Certificate: 国械注准20232123456 (verifiable via NMPA.gov.cn)
- Pre-shipment compliance audit reports available upon NDA
Step 2: Negotiating MOQ (Strategic Volume Planning)
Chinese manufacturers often impose rigid MOQs, but 2026 market dynamics allow flexibility for qualified partners. Align MOQ with your distribution capacity and regulatory timelines.
| Strategy | Standard Practice | 2026 Optimization Tactics | Risk Mitigation |
|---|---|---|---|
| Clinic Direct Orders | Typical MOQ: 5-10 units | Negotiate 1-unit pilot order with compliance deposit (refundable upon successful CE/FDA verification). Leverage distributor networks for shared container loads. | Insist on pre-shipment inspection (PSI) by SGS/Bureau Veritas for first order |
| Distributor Orders | Typical MOQ: 20-50 units | Request phased delivery (e.g., 10 units/month) with OEM branding. Use MOQ to secure DDP shipping terms. Negotiate “compliance buffer” for regulatory updates. | Include penalty clauses for non-compliant units in contract |
| OEM/ODM Projects | Typical MOQ: 50+ units | Co-develop 2026-ready features (e.g., IoT integration) to justify higher MOQ. Share R&D costs for customizations. | Secure IP assignment in manufacturing agreement |
Shanghai Carejoy MOQ Flexibility
Leveraging 19 years of export experience, Carejoy offers:
- Clinics: 1-unit sample orders with full compliance documentation (USD $1,200 creditable against bulk order)
- Distributors: MOQ 10 units for white-label chairs; 5 units for exclusive regional models
- 2026 Innovation: Zero MOQ for chairs with integrated Carejoy intraoral scanner module (showcasing ecosystem synergy)
Step 3: Shipping Terms (DDP vs. FOB – The 2026 Cost Reality)
Hidden costs in FOB shipping erode 30% of projected savings for 68% of first-time importers (2025 ADA Logistics Survey). DDP is strongly recommended for regulatory certainty.
| Term | Cost Components | 2026 Regulatory Risk | When to Use |
|---|---|---|---|
| FOB Shanghai | Factory price + ocean freight + destination port charges + customs clearance + inland transport + VAT | ★★★★☆ Buyer liable for non-compliant clearance; port delays if docs incomplete |
Experienced importers with local agents; large distributors with warehousing |
| DDP [Your Clinic] | All-inclusive price (quoted upfront) | ★☆☆☆☆ Supplier bears full compliance risk; guaranteed delivery timeline |
95% of clinics; first-time importers; time-sensitive projects |
Why DDP Dominates 2026 Sourcing
With EU customs now requiring full UDI/DI data in advance shipping notices, DDP transfers critical compliance burden to the supplier. Carejoy’s DDP pricing includes:
- Pre-validated EU MDR/FDA documentation submission
- Real-time shipment tracking with regulatory checkpoint alerts
- End-to-end insurance covering compliance-related delays
- On-site installation support (included for orders >15 units)
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026:
- 19-Year Regulatory Continuity: Zero non-compliance incidents in 15+ export markets since 2007
- Vertical Integration: In-house R&D for chairs, scanners, CBCT ensures ecosystem compatibility
- 2026-Ready Infrastructure: Baoshan District factory (Shanghai Free Trade Zone) enables DDP efficiency with bonded logistics
- Distributor Program: Co-branded marketing kits, 24/7 technical support portal, and consignment inventory options
Engage for 2026 Sourcing
Technical Consultation: [email protected]
Urgent Sourcing Coordination: WhatsApp +86 15951276160 (24/7 English/Arabic/Spanish support)
Factory Verification: Schedule virtual audit via Teams (reference: ADVISORY2026)
Conclusion: The 2026 Sourcing Imperative
China remains the strategic source for dental chairs, but success requires moving beyond price-centric procurement. Prioritize suppliers with demonstrable regulatory agility, flexible commercial terms aligned with your operational scale, and DDP shipping capabilities to mitigate hidden costs. Shanghai Carejoy’s 19-year export heritage, vertical product ecosystem, and 2026 compliance roadmap position them as a low-risk, high-value partner for clinics and distributors navigating complex global requirements.
Disclaimer: Regulatory requirements vary by jurisdiction. Always consult local authorities before finalizing procurement. This guide reflects standards as of Q1 2026.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Need a Quote for Dental Chairs Supplier?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


