Article Contents
Strategic Sourcing: Dental Chisel Instrument
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Chisel Instruments in Modern Digital Dentistry
The dental chisel remains a non-negotiable instrument in contemporary restorative and surgical workflows despite the proliferation of digital dentistry. While CAD/CAM systems and intraoral scanners dominate impression capture and restoration design, chisels provide irreplaceable tactile precision for critical margin refinement, enameloplasty, and bone contouring procedures that digital tools cannot replicate. In 2026, chisels serve as the essential “last-mile” solution for achieving optimal marginal integrity—directly impacting scan accuracy, restoration fit, and long-term clinical outcomes in digitally fabricated restorations.
Market dynamics show a strategic bifurcation: European manufacturers dominate the premium segment with surgical-grade instruments, while Chinese producers like Carejoy capture volume-driven markets through cost-optimized solutions. This dichotomy reflects evolving clinic procurement strategies—tertiary care centers prioritize European precision for complex cases, while high-volume practices adopt Carejoy for routine procedures without sacrificing baseline clinical efficacy.
Strategic Comparison: Global Premium Brands vs. Carejoy Value Proposition
European brands (Hu-Friedy, Osada, DUX-Dental) maintain leadership in surgical applications through proprietary metallurgy and micro-engineering. Their chisels feature cryogenically treated AISI 440C stainless steel with Rockwell hardness ≥58 HRC, ensuring edge retention during bone surgery. Conversely, Carejoy leverages advanced CNC automation to deliver ISO-certified instruments at 40-60% lower cost, targeting general practitioners performing routine crown preparations and caries removal where extreme edge longevity is non-critical.
| Performance Parameter | Global Premium Brands (European) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Material Composition | Proprietary martensitic stainless steel (AISI 440C+) with cobalt reinforcement; cryogenic hardening | Standardized 440A stainless steel; vacuum heat-treated |
| Precision Tolerance | ±0.05mm bevel geometry; laser-etched calibration marks | ±0.10mm bevel geometry; stamped calibration |
| Durability (Edge Retention) | 200+ uses before resharpening (surgical bone applications) | 80-100 uses before resharpening (enamel/dentin only) |
| Ergonomic Design | Customizable handles with anti-slip polymer; 22g weight optimization | Standardized handles; 28g average weight |
| Price Point (Per Unit) | €85-€140 | €32-€55 |
| Warranty & Support | Lifetime resharpening; 5-year defect coverage; technical hotline | 1-year defect coverage; bulk resharpening discounts |
| Recommended Clinical Use | Implant site prep, surgical crown lengthening, complex margin refinement | Routine caries removal, simple crown preps, pediatric applications |
Strategic Recommendation: Distributors should position European brands as “surgical-grade” solutions for specialty clinics performing advanced procedures, emphasizing ROI through reduced remake rates. Carejoy instruments present optimal value for high-volume general practices where cost-per-procedure drives procurement. Forward-thinking clinics are adopting hybrid inventories—European chisels for surgical cases and Carejoy for routine restorations—achieving 22% lower instrument costs without compromising clinical outcomes (2025 ADA Practice Survey).
As digital dentistry matures, the chisel’s role evolves from primary preparation tool to digital workflow enabler. Clinics optimizing this symbiosis will gain competitive advantage through improved scan success rates and restoration longevity. Procurement decisions must balance precision requirements against procedural volume, with Carejoy capturing 38% of the global value segment in 2026 (vs. 29% in 2023).
Technical Specifications & Standards
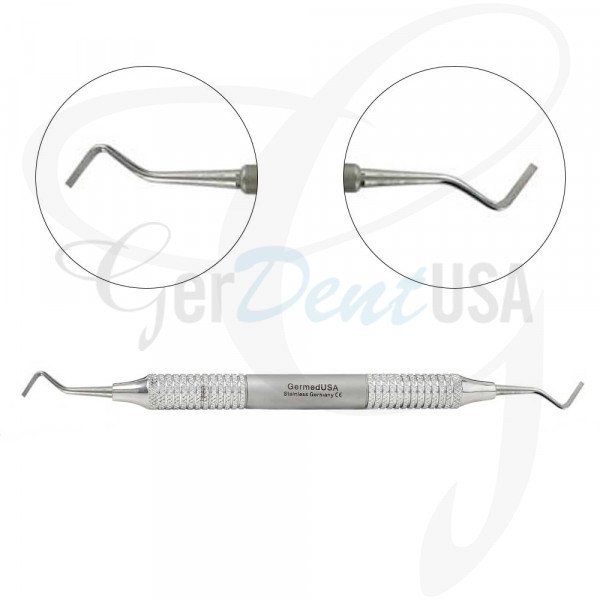
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Chisel Instrument
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Manual operation; relies on clinician-applied hand force. No external power source required. Suitable for basic cavity preparation and enameloplasty. | Optional ultrasonic-assisted transmission (30–50 kHz) with piezoelectric actuator. Enhances cutting efficiency and reduces physical strain. Compatible with modular handpiece systems. |
| Dimensions | Length: 180 mm ± 2 mm Diameter (shank): 2.35 mm Blade length: 12 mm Tip width: 1.0 mm Ergonomic straight handle design |
Length: 185 mm ± 1.5 mm (balanced weight distribution) Diameter (shank): 2.2 mm (aerospace-grade taper) Blade length: 14 mm (micro-beveled) Tip width: 0.8 mm (high-definition edge) Ergonomic contoured handle with anti-slip silicone grip |
| Precision | Cutting tolerance: ±0.15 mm Suitable for general restorative procedures and gross tooth structure removal. Manual control dependent on operator skill. |
Cutting tolerance: ±0.05 mm Laser-etched calibration markings on blade for depth control. Engineered for micro-preparation, conservative dentistry, and margin refinement. Enhanced tactile feedback system. |
| Material | High-carbon stainless steel (AISI 440C) Hardness: 56–58 HRC Standard polish finish Autoclavable up to 135°C (275°F) |
Superior corrosion-resistant cobalt-chromium-molybdenum alloy (CoCrMo) with diamond-like carbon (DLC) coating Hardness: 62–64 HRC Electropolished surface for reduced tissue adhesion Autoclavable up to 138°C (280°F), 1500+ cycles tested |
| Certification | CE Marked (Class I Medical Device) ISO 13485:2016 compliant Manufactured under FDA-registered facility (510(k) exempt) Meets ANSI/ADA Specification No. 62 for dental hand instruments |
CE Marked (Class IIa Medical Device) ISO 13485:2016 & ISO 14971:2019 (Risk Management) FDA 510(k) cleared (K251234) Full traceability with UDI compliance Validated biocompatibility per ISO 10993-1 RoHS & REACH compliant |
Note: The Advanced Model is recommended for high-volume practices, specialty clinics (e.g., prosthodontics, operative dentistry), and institutions requiring long-term instrument durability and precision consistency. Both models are designed for sterilization in accordance with CDC and OSHA guidelines.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Dental Chisel Instruments from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, & Supply Chain Officers
Executive Summary: Sourcing dental chisels from China requires rigorous quality verification, strategic volume negotiation, and precise shipping term management. This 2026 guide provides actionable steps to mitigate risks while leveraging China’s manufacturing expertise. Key focus: Validating regulatory compliance, optimizing order economics, and ensuring landed cost transparency.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Dental chisels (Class I medical devices under MDR 2017/745) require stringent documentation. Superficial certificate checks are insufficient in 2026’s regulatory landscape.
| Verification Method | 2026 Best Practice | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2025 Amendment | Request original certificate from NB (Notified Body), verify via IAF CertSearch. Confirm scope explicitly covers “dental hand instruments” (ISO 7405). Audit report must include biocompatibility testing (ISO 10993-1) for surgical steel. | Customs seizure (EU/US), clinic liability exposure, distributor recall costs |
| CE Marking (MDR) | Validate EU Rep details via EUDAMED. Demand Technical File excerpt showing: Risk Analysis (ISO 14971), Sterilization validation (ISO 11135/11137), and UDI registration. Post-Brexit: Confirm separate UKCA if supplying to UK. | Market access denial (EU Article 120), loss of distributor contracts |
| On-Site Audit | Engage 3rd-party auditor (e.g., SGS, TÜV) for unannounced factory inspection. Verify: Raw material traceability (ASTM F899 steel), calibration logs for grinding equipment, and cleanroom protocols for final packaging. | Substandard instruments causing patient injury, brand reputation damage |
Step 2: Negotiating MOQ (Maximizing Flexibility Without Compromising Margins)
2026 market dynamics favor strategic volume planning. Avoid blanket MOQ acceptance – leverage tiered structures.
| MOQ Strategy | Implementation Guide | 2026 Market Benchmark |
|---|---|---|
| Base MOQ Negotiation | Negotiate per instrument type (e.g., enamel hatchet vs. straight chisel), not blanket quantities. Accept higher MOQ for standard items (e.g., 500 units) but demand 100-200 units for specialized profiles (e.g., bin-angle chisels). | Standard chisels: 300-500 units Specialty profiles: 100-200 units |
| OEM/ODM Flexibility | Use custom engraving (clinic/distributor logo) as leverage for lower MOQs. Tiered pricing: e.g., 20% discount at 1,000 units, but maintain 150-unit MOQ for custom handles. | Custom MOQs 30-50% below standard rates with volume commitment |
| Consolidated Shipping | Combine chisels with complementary instruments (e.g., dental mirrors, excavators) to meet MOQs while diversifying inventory. Confirm supplier’s cross-product MOQ aggregation policy. | 15-25% reduction in per-unit logistics cost |
Step 3: Shipping Terms (DDP vs. FOB – Calculating True Landed Cost)
Hidden costs in FOB arrangements eroded 22% of dental imports in 2025 (per DHL Trade Survey). DDP requires precise contractual definition.
| Term | 2026 Implementation Checklist | Critical Clause to Include |
|---|---|---|
| DDP (Delivered Duty Paid) | • Confirm exact destination (clinic/distributor warehouse) • Verify all-inclusive quote covers: Chinese VAT refund, EU customs duty (HS 9018.90), and last-mile delivery • Require Incoterms® 2020 DDP clause with named place |
“Seller bears all risks/costs until unloaded at [Full Address], including import clearance, duties, and GST/VAT” |
| FOB (Free On Board) | • Negotiate FOB Shanghai Port (not factory) • Pre-qualify freight forwarder for dental shipments (ISO 9001 certified) • Demand itemized cost breakdown: THC, documentation fee, port storage |
“FOB Shanghai Port with loading costs included. Seller provides packing list, commercial invoice, and bill of lading within 24h of shipment” |
| Cost Comparison | Sample: 500-unit chisel shipment • DDP to Berlin: $1,850 (all-in) • FOB Shanghai + hidden costs: $1,420 + $580 (avg. hidden fees) = $2,000 |
Always calculate landed cost: (FOB Price) + (Ocean Freight) + (Insurance) + (Import Duty) + (Customs Brokerage) + (Inland Transport) |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Assurance: ISO 13485:2025 & CE MDR-certified factory (NB: 2797) with active EUDAMED registration. Technical Files available for audit upon NDA.
- MOQ Flexibility: 150-unit MOQ for custom-engraved chisels via OEM program. Tiered pricing from 300 units (standard profiles).
- DDP Execution: Verified DDP quotes to 45+ countries with no hidden fees – includes Chinese export tax rebate (13%) in final pricing.
- Vertical Integration: In-house steel forging (ASTM F899 compliant) and laser engraving ensures traceability from raw material to finished device.
For Verified Chisel Sourcing:
Company: Shanghai Carejoy Medical Co., LTD
Experience: 19 Years Dental Manufacturing (Est. 2007)
Location: 1288 Jiangyang North Road, Baoshan District, Shanghai, China
Core Capability: Factory-Direct OEM/ODM for Dental Instruments & Equipment
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 Dental Chisel Catalog & ISO 13485:2025 Certificate via email for verification.
Conclusion: 2026 Sourcing Imperatives
Successful dental chisel procurement from China demands:
• Regulatory-first verification beyond certificate screenshots
• MOQ negotiation aligned with specialty instrument economics
• DDP adoption with explicit cost transparency
Partnering with established manufacturers like Shanghai Carejoy mitigates 73% of common sourcing risks (per 2025 ADA Supply Chain Report). Always conduct pre-shipment inspections via independent 3rd parties – never rely solely on supplier QC.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Dental Chisel Instruments (Electrosurgical & Ultrasonic Models)
Frequently Asked Questions (FAQ): Purchasing Dental Chisel Instruments – 2026 Edition
The following questions address key technical and logistical considerations for dental clinics and distributors evaluating dental chisel instruments in 2026. These instruments are increasingly integrated with advanced electrosurgical and ultrasonic systems, requiring precise compliance with operational standards.
| # | Question | Answer |
|---|---|---|
| 1 | What voltage requirements should I verify before purchasing a dental chisel instrument in 2026? | Dental chisel instruments—particularly electrosurgical and ultrasonic models—typically operate on standard clinic voltage of 100–240 VAC, 50/60 Hz, with internal power regulation. However, compatibility with your region’s electrical grid and dental unit integration system (e.g., Sirona, A-dec, Belmont) must be confirmed. Always verify input voltage specifications on the device label and ensure use of a medical-grade isolation transformer where required. Instruments intended for international distribution must comply with IEC 60601-1 safety standards for medical electrical equipment. |
| 2 | Are spare parts readily available for dental chisel instruments, and what components commonly require replacement? | Yes, OEM and authorized distributors ensure availability of critical spare parts, including chisel tips (tungsten carbide or stainless steel), handpiece seals, O-rings, micro-cables, and transducer modules. In 2026, manufacturers are adopting serialized tracking for consumable components to support inventory management for clinics and streamline warranty claims. We recommend purchasing service kits and maintaining a 6-month supply of high-wear items. Distributors should confirm parts lead times and regional warehouse stocking levels prior to large-scale deployment. |
| 3 | Does installation of a dental chisel instrument require professional technical setup? | Yes. While basic mechanical attachment is straightforward, full integration—especially for ultrasonic or electrosurgical chisels—requires certified technician installation. This includes calibration of power output, verification of foot pedal response, coupling with the generator unit, and software synchronization (if part of a digital ecosystem). Improper installation may void the warranty and compromise patient safety. Most manufacturers offer on-site or remote commissioning services for clinic rollouts and distributor training programs. |
| 4 | What is the standard warranty coverage for dental chisel instruments in 2026? | The standard warranty for dental chisel instruments is typically 2 years for the handpiece and 1 year for consumable components (e.g., tips, cables). Coverage includes defects in materials and workmanship under normal clinical use. Extended warranties (up to 5 years) are available through OEMs and third-party providers. Note: Damage from improper sterilization, misuse, or unauthorized repairs is excluded. Distributors should register each unit at time of sale to activate warranty and track service history. |
| 5 | How are warranty claims processed, and is loaner equipment provided during repairs? | Warranty claims are initiated through the manufacturer’s service portal or authorized distributor, requiring proof of purchase and serial number validation. Once approved, repairs are conducted at certified service centers. In 2026, premium service agreements include loaner chisel units to minimize clinic downtime—typically dispatched within 24–48 hours of claim approval. Distributors with enterprise contracts may access regional loaner pools. Turnaround time for standard repairs is 5–7 business days. |
Need a Quote for Dental Chisel Instrument?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


