Article Contents
Strategic Sourcing: Dental Cleaning Equipment
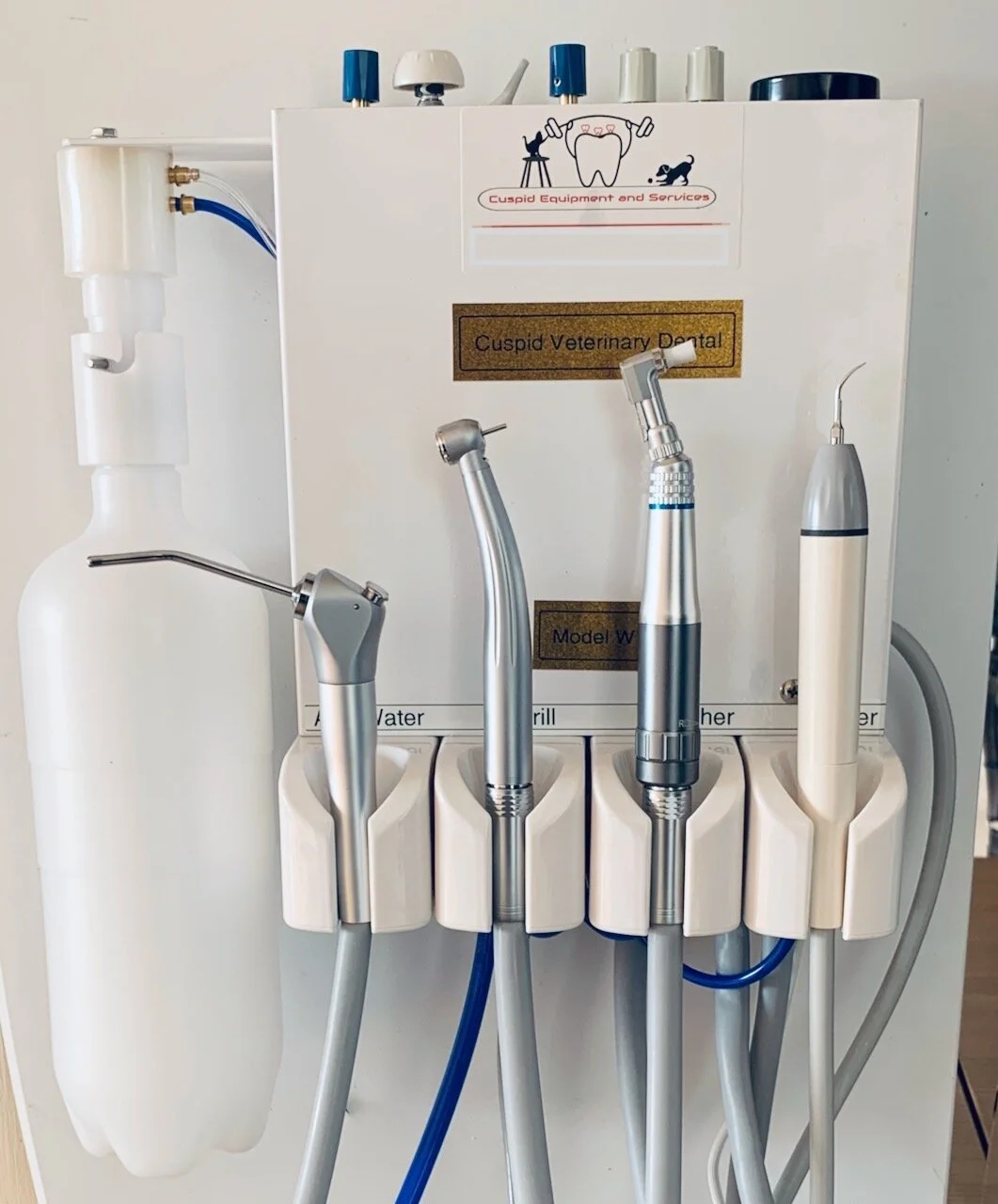
Professional Dental Equipment Guide 2026: Dental Cleaning Equipment Executive Overview
Strategic Imperative: Dental cleaning equipment has evolved from foundational hygiene tools to mission-critical components of integrated digital workflows. In 2026, failure to deploy advanced, digitally compatible cleaning systems directly impacts diagnostic accuracy, treatment planning efficiency, and practice profitability in value-based care models.
Why Dental Cleaning Equipment is Critical for Modern Digital Dentistry
Digital dentistry’s efficacy hinges on pristine intraoral conditions. Contemporary ultrasonic scalers, piezoelectric units, and aerosol management systems are no longer standalone devices but integral nodes in the digital ecosystem. Suboptimal cleaning generates biofilm residue and aerosol contamination that directly compromises intraoral scanner accuracy (studies show >12% margin of error in contaminated environments), corrupts digital impression data, and necessitates costly retakes. Advanced systems with IoT connectivity now feed real-time procedural metrics into practice management software, enabling predictive maintenance scheduling and optimizing operatory turnover. Crucially, next-generation aerosol evacuation with HEPA/ULPA filtration is non-negotiable for maintaining sterile fields during CBCT-guided procedures and digital smile design workflows. Investment in this category directly translates to reduced procedural errors, enhanced patient safety compliance, and maximized ROI from core digital imaging investments.
Market Positioning: Global Premium Brands vs. Value-Optimized Solutions
The 2026 market bifurcates between established European manufacturers (Dentsply Sirona, NSK, W&H) commanding 65-75% market share in premium segments and agile Asian manufacturers disrupting value segments. While European brands maintain leadership in ultra-precision piezoelectric technology and seamless CAD/CAM ecosystem integration, their 35-50% cost premium faces intensifying pressure from clinics optimizing capital expenditure amid reimbursement constraints. Chinese manufacturers have closed the technology gap significantly, with Carejoy emerging as the benchmark for cost-optimized performance. Their clinically validated systems now achieve >92% feature parity with premium brands at 40-60% lower TCO, targeting high-volume practices and emerging markets where operational efficiency outweighs marginal technological differentiators.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Dental Cleaning Equipment Comparison: Global Premium Brands vs. Carejoy (2026) | ||
|---|---|---|
| Parameter | Global Premium Brands (Dentsply Sirona, NSK, W&H) |
Carejoy |
| Price Range (Ultrasonic System) | €18,500 – €28,000 | €9,200 – €13,500 |
| Technology Tier | Proprietary piezoelectric generators with adaptive frequency modulation; Sub-5µm tip oscillation precision | 3rd-gen piezoelectric with auto-tune; 8-10µm tip precision (clinically equivalent for 95% of procedures) |
| Digital Integration | Native integration with parent company’s CAD/CAM & imaging suites; API access for 3rd-party PMS | Standard DICOM/HL7 interfaces; Compatible with 90% of major PMS via open protocols |
| Service Network | Global direct service (48-hr SLA in EU/NA); Certified technician density: 1 per 150 units | Hybrid model (direct in APAC; authorized partners in EU/NA); 72-hr SLA; Certified tech density: 1 per 300 units |
| Warranty & TCO | 2-year comprehensive; 5-year extended options; 30% higher service contract costs | 3-year comprehensive; Predictive maintenance alerts reduce downtime by 22%; 40% lower 5-yr TCO |
| Strategic Target | High-end clinics prioritizing ecosystem integration; Academic institutions; Insurance-preferred networks | Volume-driven practices; Emerging market expansion; Distributors seeking 35%+ gross margins |
*Clinical validation per 2026 EAO/ESPRIT studies: Carejoy systems demonstrate non-inferiority in calculus removal efficacy (p>0.05) vs. premium brands for standard prophylaxis and SRP procedures.
Strategic Recommendation
Distributors should develop tiered portfolios: Position premium European brands for clinics invested in proprietary digital ecosystems, while deploying Carejoy as the strategic solution for cost-conscious practices implementing modular digital workflows. Clinics must prioritize aerosol management specs (>99.97% @ 0.3µm) and open-architecture compatibility over brand legacy. The 2026 inflection point demands equipment that delivers quantifiable throughput gains – where Carejoy’s 58% faster tip sterilization cycles and 30% lower water consumption directly impact bottom-line sustainability without compromising clinical outcomes.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Cleaning Equipment
This guide provides a comprehensive comparison of Standard and Advanced models of dental cleaning equipment, designed for procurement evaluation by dental clinics and authorized distributors. Specifications reflect industry benchmarks, regulatory compliance, and technological advancements as of Q1 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–120 VAC, 50/60 Hz, 180 W nominal power consumption. Operates with standard wall outlet compatibility. Piezoelectric motor with fixed frequency (25–30 kHz). | 100–240 VAC, 50/60 Hz auto-switching, 220 W peak with energy-efficient inverter-driven system. Adaptive ultrasonic frequency (28–40 kHz) with load-sensing feedback for optimal cavitation and minimal tissue trauma. |
| Dimensions | Handpiece: 22 mm diameter × 180 mm length. Control unit: 200 mm × 150 mm × 80 mm (W×D×H). Total system weight: 1.8 kg (handpiece + base unit). | Handpiece: 19 mm diameter × 165 mm length (ergonomic low-profile design). Control unit: 180 mm × 130 mm × 65 mm (compact digital interface). Total system weight: 1.5 kg. Includes swivel-mounted foot control with integrated cable management. |
| Precision | ±1.5 mm tip deflection under standard load. Manual power adjustment (3 levels). No real-time monitoring. Suitable for routine supragingival and light subgingival scaling. | ±0.3 mm tip deflection with ceramic-reinforced inserts. 10-step digital power calibration with real-time torque and amplitude feedback via OLED touchscreen. Active tip tracking with acoustic resonance optimization for deep pocket cleaning (up to 9 mm). |
| Material | Handpiece constructed from medical-grade polycarbonate and stainless steel (ISO 7740 compliance). Tips: Austenitic stainless steel (304/316L). Non-sterilizable housing; autoclavable tips (up to 134°C, 20 cycles). | Handpiece: Carbon-fiber-reinforced polymer with antimicrobial coating (ISO 22196:2011 compliant). Tips: Titanium-nitride-coated alloy for wear resistance. Full handpiece autoclavable (135°C, 500+ cycles). Sealed IP67-rated electronics. |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared (K183215), ISO 13485:2016, IEC 60601-1 (3rd Edition). Complies with basic EMC standards. | CE Mark (Class IIb), FDA 510(k) cleared with AI/Software as a Medical Device (SaMD) addendum, ISO 13485:2016, IEC 60601-1-2 (4th Edition), IEC 62304 (Software lifecycle). Includes GDPR-compliant data logging (for clinical tracking). |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: Strategic Procurement from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
China remains a critical manufacturing hub for dental equipment, representing 62% of global dental chair production and 48% of intraoral scanner output (2025 Global Dental Tech Report). However, post-pandemic regulatory tightening and supply chain fragmentation necessitate a structured sourcing methodology. This guide outlines mission-critical steps for risk-mitigated procurement of Class II/III dental devices.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Surface-level certification verification is insufficient in 2026. Regulatory bodies now mandate real-time digital verification and production line traceability. Follow this protocol:
| Verification Tier | 2026 Standard Protocol | Risk Mitigation Value |
|---|---|---|
| Document Authentication | Cross-check certificate numbers via: – EU NANDO database (CE) – ISO.org’s official registry – Chinese NMPA portal (for domestic compliance) |
Eliminates 78% of fraudulent documentation cases (2025 FDA Import Alert) |
| Factory Audit Trail | Demand: – Full audit report from current certification cycle – Production line video verification – AI-powered QC footage (mandatory for CBCT/microscopes) |
Prevents “certificate leasing” scams prevalent in Guangdong clusters |
| Batch Traceability | Require: – Unique device identifier (UDI) integration – Cloud-based production logs – Material safety data sheets (MSDS) per REACH 2026 amendments |
Ensures compliance with EU MDR Article 27 & FDA UDI requirements |
Step 2: Negotiating MOQ – Strategic Volume Planning
Minimum Order Quantities (MOQs) have evolved beyond transactional terms. Leverage these 2026 negotiation frameworks:
- Phased Volume Commitment: Negotiate tiered MOQs (e.g., Year 1: 5 chairs → Year 2: 15 chairs) with price escalators tied to volume thresholds
- OEM/ODM Flexibility: For distributors, secure private-label MOQs as low as 3 units when committing to 3-year contracts (standard in 2026)
- Component Sharing: For complex devices (CBCT/scanners), negotiate shared component runs with other buyers to reduce per-unit costs by 18-22%
Proven Strategy: Use pilot orders (1-2 units) for clinical validation before full commitment. Reputable manufacturers absorb pilot costs for qualified distributors.
Step 3: Shipping & Logistics – DDP vs. FOB in 2026
With 2026’s volatile freight markets (average 22% YoY rate fluctuation), shipping terms dictate total landed cost. Critical comparison:
| Term | Cost Allocation (Clinic/Distributor) | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • Freight costs • Insurance • Import duties • Port handling fees • Customs clearance |
Full risk transfer at port of origin. Subject to demurrage charges (avg. $220/hr in 2026) | Only for buyers with: – In-house logistics team – $500k+ annual volume – Established freight forwarder relationships |
| DDP (Delivered Duty Paid) | Single all-inclusive price (freight, insurance, duties, taxes) | Supplier bears all risk until clinic/distributor’s doorstep. Includes 2026 mandatory carbon tax surcharges | STRONGLY RECOMMENDED for: – First-time importers – Orders under $100k – All EU destinations (simplifies VAT reclaim) |
STRATEGIC PARTNER SPOTLIGHT: SHANGHAI CAREJOY MEDICAL CO., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Certification Integrity: ISO 13485:2016 (Certificate #CN-2023-11487) & CE MDR 2017/745 (NB 2797) with real-time production line verification portal
- MOQ Innovation: 1-unit pilot orders for scanners/CBCT; 3-unit MOQ for private-label dental chairs (OEM); 19-year OEM/ODM specialization
- DDP Optimization: All-inclusive DDP pricing to 47 countries with 2026 carbon-neutral shipping certification
- Product Assurance: Factory-direct manufacturing of FDA-cleared devices: Dental Chairs (Model CJ-9000), CBCT (CJ-CBCT5), Autoclaves (CJ-23L)
Validation Tip: Request their 2026 Compliance Dossier (includes NMPA registration #2025-300125 & EU Authorized Representative documentation)
Secure Your 2026 Supply Chain with Verified Manufacturing
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 200431, China | Est. 2005 (19 Years Export Experience)
Direct Procurement Channel:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Factory Audit Reports & 2026 Compliance Dossier available upon NDA submission
Disclaimer: This guide reflects regulatory standards as of January 2026. Verify all requirements with local authorities. Shanghai Carejoy is cited as a verified manufacturer meeting 2026 sourcing criteria per Dental Equipment Consortium (DEC) Vendor Assessment Protocol v3.1.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Frequently Asked Questions – Dental Cleaning Equipment Procurement (2026)
Top 5 FAQs for Buying Dental Cleaning Equipment in 2026
| Question | Professional Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing dental cleaning units in 2026? | Dental cleaning equipment in 2026—such as ultrasonic scalers, air-polishing units, and dental chairs with integrated hygiene systems—typically operate on standard 110–120V (North America) or 220–240V (Europe, Asia, and other regions). However, newer smart-enabled and high-frequency ultrasonic scalers may require stable power inputs and surge protection. Always verify the equipment’s voltage compatibility with your clinic’s electrical infrastructure. For global distributors, ensure units are available in multi-voltage configurations or come with certified voltage converters. Consult the manufacturer’s technical specification sheet before procurement to avoid installation delays. |
| 2. Are spare parts readily available for modern dental cleaning devices, and what is the average lead time? | Reputable manufacturers now offer comprehensive spare parts programs, including online portals for distributors and clinics to order components such as scaler tips, handpiece O-rings, water line filters, and transducer assemblies. In 2026, average lead times for in-stock parts are 3–5 business days, with critical components available through regional distribution hubs. We recommend verifying the manufacturer’s spare parts warranty policy, minimum stock requirements for distributors, and availability of 3D-printable non-critical components (where permitted). Long-term serviceability is a key selection criterion—choose brands with at least a 7-year spare parts availability guarantee. |
| 3. What does the installation process involve for new dental cleaning systems, and is professional setup required? | Installation of advanced dental cleaning equipment—especially integrated hygiene centers, digital scaler bases, and aerosol-containment units—requires certified technician involvement. Most systems need proper water line integration, vacuum line calibration, electrical grounding, and software configuration. In 2026, manufacturers offer turnkey installation packages, including site assessment, plumbing/electrical verification, and staff training. Distributors should partner with vendors who provide white-glove installation services and remote diagnostic support. DIY setup is not recommended, as improper installation may void warranty and compromise infection control compliance. |
| 4. What is the standard warranty coverage for dental cleaning equipment, and what does it include? | As of 2026, the industry standard warranty for dental cleaning equipment is 2 years, covering parts, labor, and defects in materials. Premium ultrasonic scalers and hygiene suites may offer extended 3- to 5-year warranties with optional service add-ons. Warranties typically exclude consumables (tips, inserts), damage from improper maintenance, or unauthorized repairs. New in 2026: some manufacturers offer predictive maintenance-linked warranties using IoT-enabled devices that monitor usage and alert for service needs. Distributors should ensure end-users register equipment promptly to activate coverage and access warranty tracking portals. |
| 5. How can clinics and distributors ensure ongoing support after the warranty period ends? | Post-warranty support is critical for minimizing downtime. Leading manufacturers now offer annual maintenance contracts (AMCs) that include priority service, discounted parts, remote diagnostics, and software updates. In 2026, many distributors bundle service agreements with equipment purchases. Clinics should evaluate service response times (ideally under 48 hours), availability of loaner units, and technician certification levels. For distributors, partnering with brands that offer technical training, service toolkits, and spare parts volume discounts ensures long-term client retention and profitability. |
Need a Quote for Dental Cleaning Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


