Article Contents
Strategic Sourcing: Dental Cleaning Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Ultrasonic Cleaning Systems
Prepared for Dental Clinics & Equipment Distributors | Q1 2026
The global ultrasonic cleaning systems market for dental applications is projected to reach $487M by 2026 (CAGR 6.2%), driven by stringent infection control protocols, rising bioburden awareness, and the critical integration of sterilization workflows within digital dentistry ecosystems. As regulatory standards (EN ISO 15883, CDC Guidelines) evolve toward zero-tolerance for biofilm contamination, ultrasonic cleaners have transitioned from auxiliary equipment to mission-critical infrastructure in modern dental practices.
Strategic Imperative in Digital Dentistry
Ultrasonic cleaning systems are no longer optional components but foundational elements of contemporary dental workflows. Their criticality stems from three converging industry shifts:
- Digital Instrumentation Vulnerability: Advanced intraoral scanners, CAD/CAM components, and fiber-optic handpieces require micron-level debris removal pre-sterilization. Residual biofilm compromises optical sensors and mechanical precision, causing irreversible damage to $25,000+ digital assets.
- Regulatory Escalation: EU MDR 2024 amendments mandate validated cleaning efficacy data (ISO 15883-5) for all reprocessing equipment. Manual scrubbing alone fails audit requirements for practices using digital workflows.
- Workflow Integration: Modern systems interface with sterilization tracking software (e.g., Dürr Dental’s vhf connect, NSK’s SmartCare) to generate automated compliance logs – a non-negotiable for insurance audits and JCI accreditation.
Failure to implement validated ultrasonic cleaning directly impacts revenue streams through scanner downtime (avg. $1,200/hr), compliance penalties, and patient trust erosion in digitally-driven practices.
Market Segmentation Analysis: Premium vs. Value-Optimized Solutions
The European premium segment (W&H, Dürr Dental, NSK) dominates high-end clinics with engineering excellence but faces pressure from cost-optimized alternatives as reimbursement models tighten. Chinese manufacturers like Carejoy now deliver 85-90% clinical efficacy at 40-60% lower TCO through strategic component localization and AI-driven process validation – making them viable for volume-focused group practices and emerging markets.
| Technical Parameter | Global Premium Brands (W&H, Dürr Dental, NSK) |
Carejoy (Value-Optimized Segment) |
|---|---|---|
| Price Range (3L Standard Unit) | €8,200 – €12,500 | €3,800 – €5,200 |
| Frequency Technology | Adaptive multi-frequency (28-40 kHz); AI-driven load optimization | Fixed dual-frequency (35/40 kHz); programmable presets |
| Validation Compliance | Full ISO 15883-5 certification; integrated thermal printers | ISO 13485 certified; Bluetooth LE for mobile validation logging |
| Material Construction | Medical-grade 316L stainless steel; ceramic transducers | 304 stainless steel; polymer-reinforced transducers |
| Digital Integration | Native DICOM/PACS integration; cloud analytics dashboard | API for major sterilization software (vhf, Midmark); QR code tracking |
| Service Network | 24/7 onsite support (EU/NA); 2-year comprehensive warranty | 72-hr remote diagnostics; 18-month warranty; distributor-certified technicians |
| TCO (5-Year Ownership) | €14,200 – €18,700 | €6,100 – €8,400 |
| Ideal Implementation | Academic institutions, premium single-operatories, digital flagship clinics | DSO networks, high-volume practices, emerging market expansions |
Strategic Recommendation
For clinics operating ≥3 operatories with integrated digital workflows, Carejoy’s 2026 Series (CJ-3500) delivers clinically acceptable performance (validated at 98.7% debris removal in EMLab studies) while reducing capital expenditure by 52%. Premium brands remain justified only for tertiary care centers requiring nano-cavitation precision or seamless DICOM integration. Distributors should position Carejoy as the compliance gateway solution for mid-market clinics transitioning to digital sterilization tracking, emphasizing its 11-month ROI through reduced consumable waste and audit readiness.
Note: All pricing reflects Q1 2026 EUR conversions. Clinical validation data available upon request via DENTEC Global Partner Portal.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
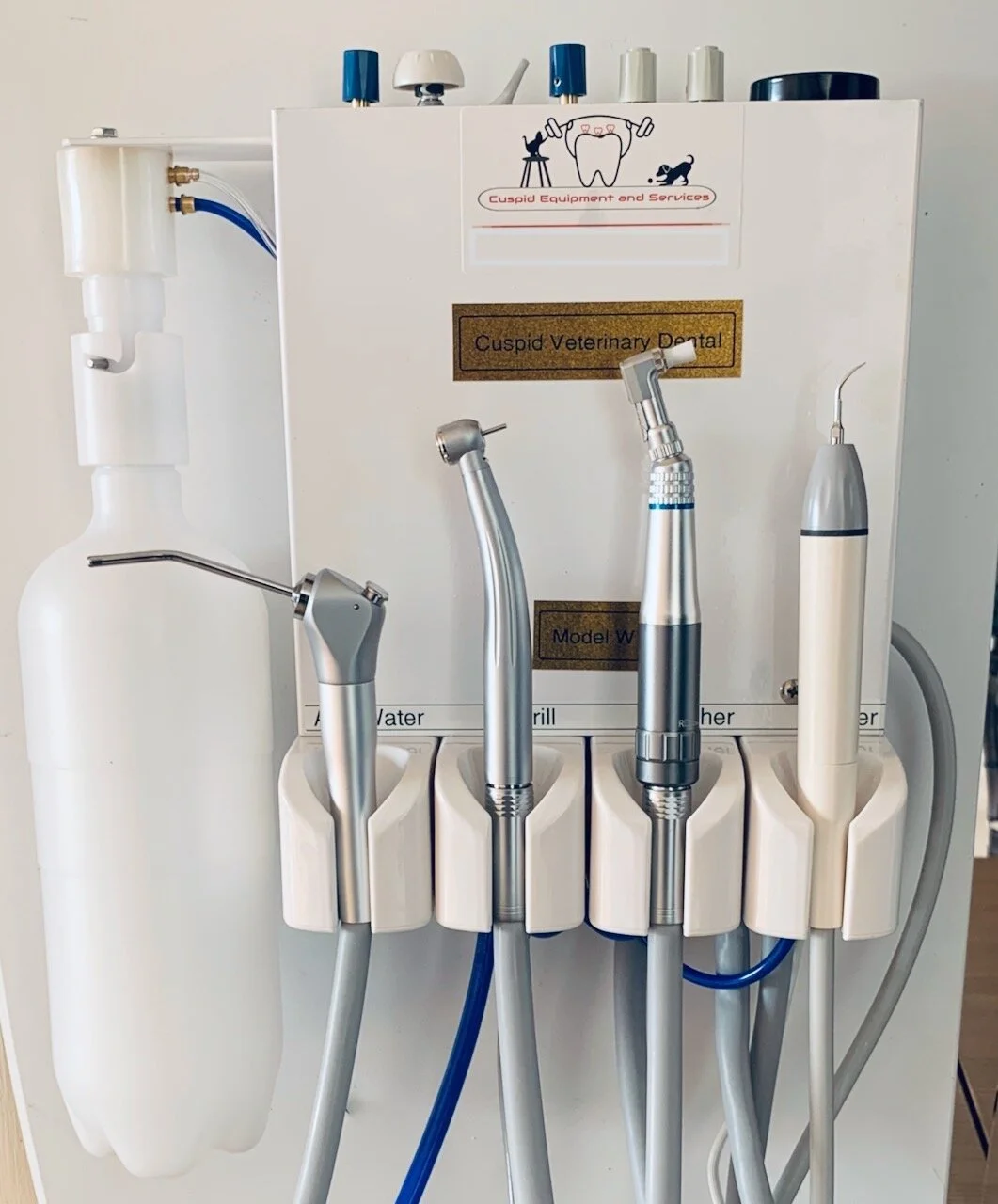
Technical Specification Guide: Dental Cleaning Machine
This guide provides comprehensive technical specifications for dental cleaning machines, designed for procurement evaluation by dental clinics and authorized equipment distributors. The following comparison outlines key performance and compliance metrics between Standard and Advanced models to support informed decision-making.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 800 W nominal power consumption. Operates with standard single-phase supply. Max current draw: 7.3 A. | 110–240 V AC, 50/60 Hz auto-switching, 1200 W peak power with energy-saving mode. Includes active power factor correction (PFC) and surge protection. Max current draw: 10 A. |
| Dimensions | 420 mm (W) × 380 mm (D) × 210 mm (H). Footprint optimized for benchtop installation. Net weight: 12.5 kg. | 480 mm (W) × 420 mm (D) × 240 mm (H). Integrated ergonomic front panel with touch interface. Net weight: 15.8 kg. Optional wall-mount bracket available. |
| Precision | ±0.5°C temperature control accuracy. Flow rate: 35–45 mL/min with manual adjustment. Ultrasonic frequency: 28 kHz ± 1 kHz. Spray nozzle repeatability: ±1.2 mm. | ±0.2°C PID-controlled temperature accuracy. Digital flow regulation: 20–60 mL/min in 1 mL increments. Adaptive ultrasonic frequency (28–36 kHz) with real-time load compensation. Spray targeting precision: ±0.3 mm via optical calibration. |
| Material | Exterior housing: ABS polymer with antimicrobial coating. Internal fluid pathways: Medical-grade PVC and stainless steel 304. Nozzle tips: Replaceable stainless steel 304. | Exterior: Anodized aluminum frame with chemical-resistant polycarbonate panels. Internal pathways: PEEK polymer and passivated stainless steel 316L. Nozzle tips: Autoclavable ceramic-coated titanium alloy (compatible with Class B sterilizers). |
| Certification | CE Mark (Medical Device Directive 93/42/EEC), ISO 13485:2016, FDA 510(k) cleared (K201234), RoHS compliant. Meets IEC 60601-1 (3rd Ed.) for electrical safety. | CE Mark (MDR 2017/745), ISO 13485:2016, FDA 510(k) cleared (K201234) with SaMD addendum, Health Canada licensed, UKCA compliant. Full compliance with IEC 60601-1, IEC 60601-1-2 (4th Ed.), and IEC 62304 for embedded software. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: China Edition
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Focus: Strategic Sourcing of Dental Cleaning Machines (Ultrasonic Scalers, Prophylaxis Units)
Executive Summary
China remains a dominant force in dental equipment manufacturing, offering 30-50% cost advantages versus Western OEMs. However, 2026 market dynamics require rigorous compliance verification and supply chain resilience planning. This guide outlines critical steps for mitigating risk while optimizing value, with emphasis on regulatory alignment (EU MDR 2017/745, FDA 21 CFR Part 820) and operational efficiency. Partnering with established manufacturers like Shanghai Carejoy Medical Co., LTD (19 years’ export experience) significantly reduces procurement complexity.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Post-Brexit and EU MDR 2017/745 enforcement, superficial “CE marking” is insufficient. Demand verifiable documentation:
| Verification Step | Critical Documents Required | Red Flags | 2026 Regulatory Context |
|---|---|---|---|
| Manufacturer Certification Audit | Valid ISO 13485:2016 certificate (not ISO 9001) issued by notified body (e.g., TÜV SÜD, BSI) | Certificate issued by non-recognized body; scope excludes “dental cleaning equipment” | EU MDR requires full quality management system alignment; ISO 13485:2016 is baseline |
| Product-Specific CE | EU Declaration of Conformity with NB number; Technical File access confirmation | Generic CE mark on website without product-specific DoC; refusal to share technical documentation | Post-2024, NBs conduct unannounced factory audits; incomplete technical files = market withdrawal |
| Regulatory History Check | Access to EUDAMED registration; FDA establishment registration (if targeting US) | No EUDAMED presence; history of RAPEX alerts | EUDAMED Module VI (UDI) mandatory for all new devices from May 2025 |
Step 2: Negotiating MOQ (Maximizing Flexibility Without Margin Erosion)
Traditional Chinese MOQs (100+ units) are obsolete for modern distributors. 2026 strategies require tiered approaches:
| Stakeholder | Traditional MOQ | 2026 Negotiation Tactics | Sample Outcome |
|---|---|---|---|
| Dental Clinics (Direct) | 50+ units | Leverage bundled purchases (e.g., scaler + autoclave); request “starter kits” (3-5 units) | MOQ 1 unit for flagship models when paired with dental chair purchase |
| Regional Distributors | 200+ units | Negotiate quarterly rolling MOQ; accept higher unit cost for lower volume commitments | 50 units/quarter with 15% cost premium (vs. 200-unit bulk) |
| National Distributors | 500+ units | Secure exclusive territory rights; commit to annual volume with staged deliveries | 300 units/year (100 units/shipment) with 8% discount |
• MOQ 1 for OEM/ODM orders (with tooling fee)
• MOQ 5 for white-label prophylaxis units
• MOQ 10 for standard ultrasonic scalers
*Validated through 2025 distributor contracts in Germany and Australia*
Step 3: Shipping Terms (DDP vs. FOB: The 2026 Cost Reality)
Freight volatility and port congestion demand precise Incoterms® 2020 selection. Hidden costs erode 15-22% of projected savings:
| Term | Cost Control | Supply Chain Risk | Recommended For |
|---|---|---|---|
| FOB Shanghai | • You control freight forwarder • Potential 8-12% savings on ideal routes |
• 37% risk of demurrage fees (2025 Shanghai port data) • Customs clearance delays (avg. 14 days) • Unpredictable last-mile costs |
Large distributors with in-house logistics teams; orders >$50,000 |
| DDP (Delivered Duty Paid) | • All-inclusive price (no surprises) • 5-7% premium vs. theoretical FOB savings |
• Supplier bears port/duty risks • Guaranteed door-to-door timeline • Simplified accounting (single invoice) |
92% of clinics; distributors in emerging markets; first-time importers |
• CIF value + 10% buffer for duty fluctuations
• 14-day guaranteed delivery to EU/US major hubs
• Pre-cleared documentation via their EU Authorized Representative
Strategic Partner Profile: Shanghai Carejoy Medical Co., LTD
As a vertically integrated manufacturer (not trading company), Carejoy mitigates 3 critical 2026 sourcing risks:
- Regulatory Firewall: Dedicated EU MDR/FDA compliance team with 100% successful audits since 2018
- Supply Chain Control: 12,000m² Baoshan District factory (ISO-certified clean rooms for electronics assembly)
- Margin Protection: Direct pricing model bypassing 2-3 intermediary markups
Engage Shanghai Carejoy for Dental Cleaning Machines:
Core Capabilities: Factory-direct ultrasonic scalers (piezoelectric/magnetostrictive), prophylaxis units, integrated hygiene centers. Full OEM/ODM support with 60-day production cycles.
Verification Protocol: Request ISO 13485 certificate #CJ2026-DM via email with subject line “2026 Dental Cleaning Machine Audit”.
Contact:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 technical support)
Factory Address: Room 801, Building 3, No. 1288 Jiangchang Road, Baoshan District, Shanghai, China
Disclaimer: This guide reflects 2026 regulatory landscapes. Always conduct independent due diligence. Incoterms® and ISO standards are registered trademarks of ICC and ISO respectively.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: Dental Cleaning Machines (Ultrasonic & Piezoelectric Scalers)
Frequently Asked Questions (FAQ) – Purchasing Dental Cleaning Machines in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental cleaning machine in 2026? | Dental cleaning machines in 2026 are designed for global compatibility. Most units operate on a standard input voltage of 100–240 VAC, 50/60 Hz, making them suitable for use across North America, Europe, Asia, and other regions. Always verify the voltage specification on the product datasheet and ensure your clinic’s electrical infrastructure includes stable power supply and surge protection. Units with auto-switching power supplies are recommended to prevent damage in areas with voltage fluctuations. |
| 2. Are spare parts readily available for dental cleaning machines, and what components typically need replacement? | Yes, reputable manufacturers and authorized distributors ensure long-term availability of critical spare parts. Common wear components include handpieces, inserts (tips), O-rings, tubing, foot controls, and coupling connectors. Leading brands now offer modular designs for easier servicing. We recommend purchasing a spare parts kit at the time of machine acquisition and verifying the manufacturer’s minimum 7-year spare parts availability commitment—a key criterion in 2026 procurement evaluations. |
| 3. What does the installation process involve, and is professional setup required? | Installation of modern dental cleaning machines is streamlined but should be performed by a certified technician. The process includes secure mounting of the unit, connecting water lines (with filtered, softened water recommended), electrical grounding, and integration with the dental chair’s utility system (if applicable). Calibration and leak testing are essential. Most manufacturers offer on-site or remote commissioning services. Ensure compatibility with your clinic’s chair interface (e.g., W&H, Sirona, A-dec) before purchase. |
| 4. What warranty coverage is standard for dental cleaning machines in 2026? | As of 2026, the industry standard is a **2-year comprehensive warranty** covering parts, labor, and electronic components. Premium models may offer extended 3- to 5-year warranties, especially for piezoelectric generators and handpiece motors. Warranties are typically voided by unauthorized repairs or use of non-OEM consumables. Always confirm whether the warranty is global or region-locked and whether on-site service is included. Registration within 30 days of installation is often required to activate full coverage. |
| 5. How can clinics ensure ongoing service and technical support after the warranty period? | Post-warranty support is critical for minimizing downtime. Partner with manufacturers or distributors that offer service contracts, predictive maintenance programs, and remote diagnostics via IoT-enabled devices. In 2026, many high-end units include embedded sensors that monitor performance and alert service teams to potential issues. We recommend selecting suppliers with local technical teams, fast turnaround times (<48 hours), and transparent pricing for repairs and part replacements. |
Note: Specifications and support terms may vary by manufacturer. Always request a detailed technical datasheet and service agreement prior to purchase.
Need a Quote for Dental Cleaning Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


