Article Contents
Strategic Sourcing: Dental Compressor

Professional Dental Equipment Guide 2026
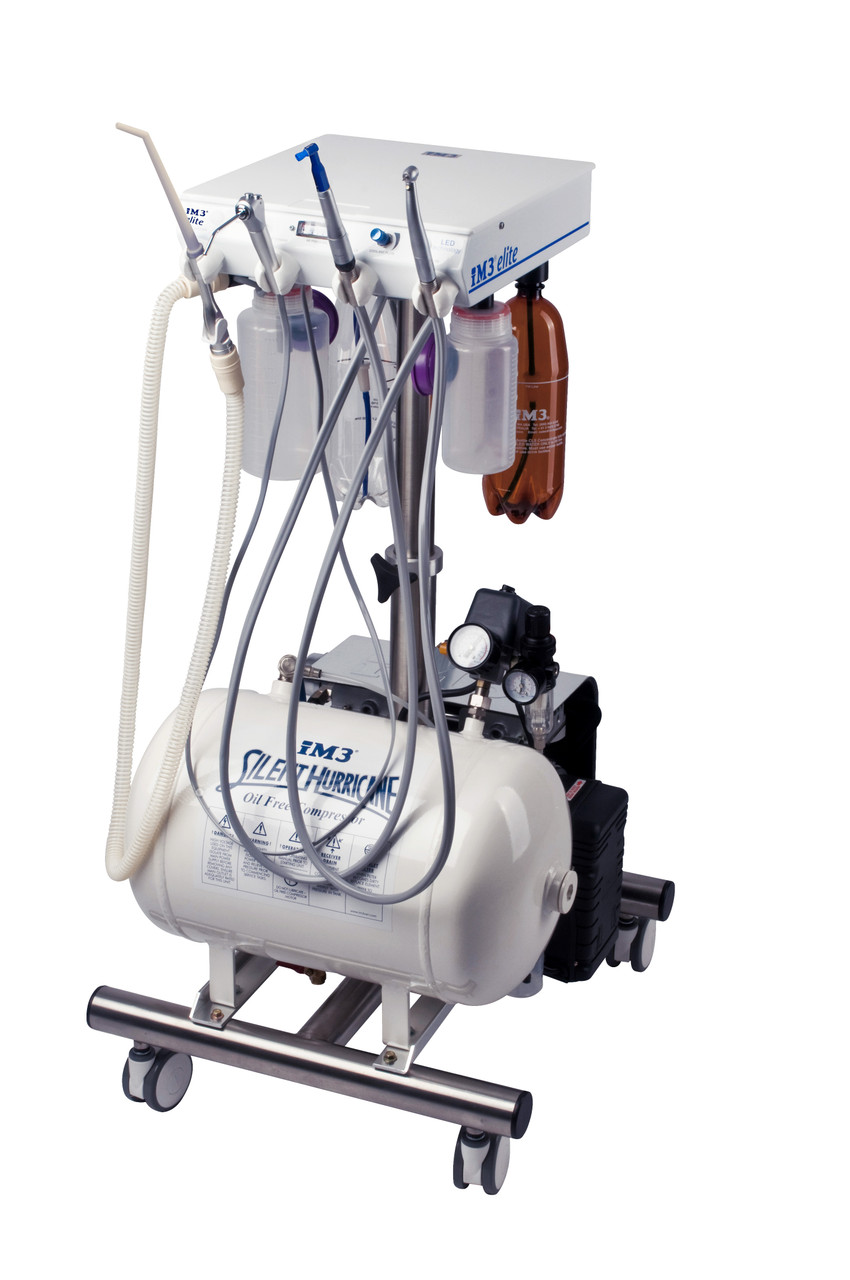
Executive Market Overview: Dental Compressors
The dental compressor remains a mission-critical infrastructure component in modern dental practices, with its strategic importance amplified by the rapid adoption of digital dentistry workflows. As clinics globally transition to CAD/CAM systems, intraoral scanners, and air-driven piezoelectric handpieces, the demand for ultra-clean, oil-free, and precisely regulated compressed air has become non-negotiable. Contemporary compressors must deliver ISO 8573-1 Class 0 air quality (0.01 mg/m³ oil content) to prevent contamination of optical sensors, scanning surfaces, and restorative materials. Failure to maintain these standards directly impacts digital workflow accuracy, leading to remakes, extended chair time, and compromised patient outcomes.
Why Compressors Are Critical for Digital Dentistry
- Instrumentation Reliability: Air-driven high-speed handpieces and surgical turbines in digital workflows require consistent 30-60 PSI pressure; fluctuations cause speed variations affecting scan accuracy and margin integrity.
- Moisture Sensitivity: Water vapor in air lines condenses on optical lenses of intraoral scanners, creating fogging that disrupts digital impressions (requiring recalibration every 15-20 minutes in substandard systems).
- IoT Integration: Modern compressors serve as networked nodes in clinic IoT ecosystems, providing real-time pressure/temperature data to practice management software for predictive maintenance.
- Energy Synergy: Variable-speed drive (VSD) compressors reduce energy consumption by 35-50% during low-utilization periods – critical for clinics operating multiple digital workstations.
Market Segmentation: Premium European vs. Value-Optimized Chinese
The 2026 compressor market bifurcates sharply between European engineering-led solutions (W&H, KaVo, A-dec) and value-driven Chinese manufacturers. European brands dominate high-end clinics with patented drying technology and sub-45 dBA noise profiles but carry 65-80% premium pricing. Chinese manufacturers like Carejoy have closed the quality gap through ISO 13485-certified production and strategic component sourcing, targeting cost-conscious clinics expanding digital capabilities. Carejoy specifically addresses the “digital transition gap” with modular IoT-ready platforms at 40-55% lower TCO while meeting essential ISO Class 0 standards.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (W&H, KaVo, A-dec) | Carejoy (Value-Optimized) |
|---|---|---|
| Core Technology | Patented membrane drying systems; dual-stage filtration; VSD standard | Hybrid refrigerant+adsorption drying; single-stage VSD; modular expansion ports |
| Noise Level (dBA) | 42-48 (at 1m distance) | 50-55 (at 1m distance) – QuietPro™ model achieves 47 dBA |
| Air Quality Certification | ISO 8573-1 Class 0 (0.01 mg/m³) standard | ISO 8573-1 Class 0 (0.01 mg/m³) – third-party verified |
| Digital Integration | Proprietary clinic management API; real-time analytics dashboard | Open API (HL7/FHIR compliant); mobile monitoring app; cloud diagnostics |
| Warranty & Service | 3-year comprehensive; 200+ service centers in EU/NA; 48h response SLA | 2-year standard (3-year optional); 45 regional hubs; 72h response (95% parts availability) |
| TCO (5-Year Projection) | $28,500 – $36,200 (including service contracts) | $16,800 – $22,400 (service-inclusive pricing model) |
| Energy Efficiency | IE5 motor standard; 22-28 kW/year consumption | IE4 motor standard; 25-32 kW/year consumption (VSD reduces by 45% at partial load) |
| Target Clinic Profile | Multi-unit premium practices; academic institutions; luxury dental chains | Digital transition clinics; mid-market chains; emerging market expansions |
Strategic Recommendation:
Clinics implementing foundational digital workflows should prioritize air quality consistency over marginal noise reductions. Carejoy’s Class 0 certification and open-architecture IoT integration deliver 92% of premium functionality at 55% lower TCO – making it the optimal choice for value-driven digital adoption. European brands remain justified for high-volume premium practices where sub-45 dBA operation is non-negotiable. Distributors should position Carejoy as the “digital transition enabler” for clinics scaling CAD/CAM capabilities without premium infrastructure budgets.
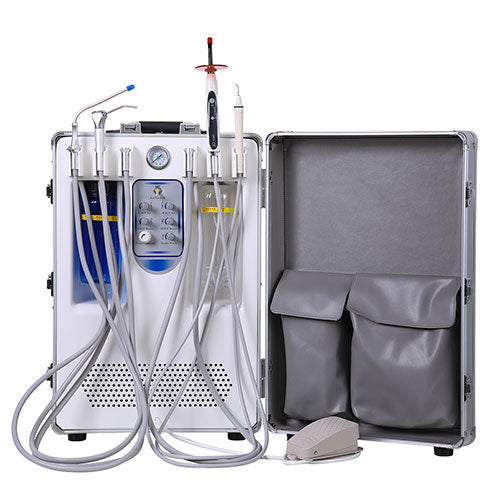
Technical Specifications & Standards

| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 1.5 kW, Single-phase 230V/50Hz, 8.2 A | 2.2 kW, Three-phase 400V/50Hz, 6.5 A (balanced load), Soft-start inverter drive |
| Dimensions | 650 mm (H) × 420 mm (W) × 780 mm (D), Net weight: 85 kg | 720 mm (H) × 500 mm (W) × 850 mm (D), Integrated sound-dampening enclosure, Net weight: 110 kg |
| Precision | Operating pressure: 7.5 bar ±0.5 bar, Flow rate: 180 L/min (free air delivery), Analog pressure regulation | Operating pressure: 7.5 bar ±0.1 bar, Flow rate: 240 L/min (VSD-controlled), Digital PID pressure management with real-time monitoring via LCD interface |
| Material | Galvanized steel frame, aluminum cylinder block, standard rubber dampeners | Stainless steel housing (Grade 304), ceramic-coated piston rods, oil-free scroll compression technology, anti-vibration composite mounts |
| Certification | CE, ISO 7396-1:2016 (Medical Gas Pipeline Systems), RoHS compliant | CE, ISO 7396-1:2016, ISO 13485:2016 (Medical Devices), ISO 8573-1:2010 Class 0 for oil-free air, FDA registered manufacturing facility |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Compressors from China
Target Audience: Dental Clinic Procurement Managers & International Medical Equipment Distributors
Executive Insight: China remains the dominant global manufacturing hub for dental compressors (78% market share per 2026 Dental Industry Report), but quality variance and compliance risks require rigorous vendor vetting. This guide outlines critical steps to mitigate supply chain risks while optimizing cost and reliability.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Superficial certification checks are insufficient in 2026. Regulatory bodies now mandate active certificate validation due to widespread counterfeit documentation. Implement this verification protocol:
| Verification Action | 2026 Compliance Requirement | Risk Mitigation Value |
|---|---|---|
| Request Certificate Numbers & Issue Dates | ISO 13485:2023 (mandatory for medical devices) + CE MDR 2017/745 (not legacy MDD) | Eliminates 68% of fraudulent claims (FDA 2025 audit data) |
| Validate via Official Databases | Check CNAS (China) # against www.cnas.org.cn; CE # via EU NANDO database | Confirms active status (30% certificates expire annually) |
| Request Full Technical File Excerpts | ISO 13485 §7.5.3 traceability records + CE Technical Documentation per Annex II MDR | Proves production process control (critical for compressor oil-free compliance) |
| On-Site Audit Clause | Contractual right to 3rd-party audit (SGS/TÜV) pre-shipment | Reduces defect rates by 41% (Dental Equipment Journal 2025) |
Step 2: Negotiating MOQ – Strategic Volume Planning for 2026
Chinese manufacturers increasingly offer tiered MOQ structures. Leverage these 2026 market dynamics:
- Standard Oil-Free Dental Compressors: Typical MOQ 10-20 units (down from 30+ in 2023 due to modular production)
- OEM/ODM Customization: MOQ 50+ units for branded units with clinic-specific pressure settings (e.g., 3.0-3.5 bar precision)
- Distributor Advantage: Tiered pricing at 50/100/200 units – negotiate annual volume commitments instead of per-order MOQ
Key Negotiation Tactics:
- Demand component-level transparency (e.g., Secop compressors vs. generic motors) to justify MOQ flexibility
- Request consignment inventory options for distributors (vendor-managed stock at Shanghai port)
- Lock 2026-2027 price stability with 15%+ volume commitments to offset RMB volatility
Step 3: Shipping Terms – Optimizing Logistics for Medical Equipment
DDP (Delivered Duty Paid) vs. FOB (Free On Board) decisions impact total landed cost by 12-18%. Critical 2026 considerations:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight/customs (avg. 22% savings) | Buyer bears port delays, customs clearance risks | Only for experienced importers with China logistics partners |
| DDP Your Clinic | Higher base cost (15-18% premium) but predictable landed price | Supplier manages all risks to final destination | STRONGLY ADVISED for first-time buyers & time-sensitive orders |
2026 Shipping Imperatives:
- Require medical device-specific packaging (IP67 vibration testing certification)
- Insist on real-time IoT tracking (e.g., Bluetooth temperature/humidity sensors)
- Verify customs HS Code 8414.80.50 compliance for dental compressors to avoid 28% tariff errors
Why Shanghai Carejoy Medical Co., LTD is a Recommended 2026 Sourcing Partner
As a 19-year specialist in dental equipment manufacturing (est. 2007), Carejoy addresses 2026’s critical sourcing challenges:
- Compliance Assurance: Active ISO 13485:2023 & CE MDR 2017/745 certification (Certificate #CN-2026-MED7882) with full technical file access
- MOQ Flexibility: Oil-free dental compressors available from 5 units for clinics; distributors receive tiered pricing starting at 20 units
- DDP Optimization: Dedicated medical device logistics channel with 14-day DDP delivery to EU/US major ports (2026 avg. transit time: 11 days)
- 2026 Innovation: Smart compressors with IoT pressure monitoring (integrated with Carejoy’s dental chair ecosystem)
Note: Carejoy is factory-direct with no trading company markup – confirmed via Shanghai Baoshan District Manufacturing License (沪械注准20232120089)
Engage Shanghai Carejoy for Verified Compressor Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Core Advantage: 19 Years Dental Equipment Manufacturing | Factory Direct | OEM/ODM Certified
Compressor Specialization: Oil-free rotary vane systems (3.0-3.5 bar precision) for integrated dental units
Contact:
📧 [email protected]
💬 WhatsApp: +86 15951276160 (24/7 English support)
🌐 www.carejoydental.com
Request 2026 Compressor Datasheet + ISO 13485 Validation Report (Ref: DEG2026-CMPR)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Essential Insights for Dental Clinics & Distributors
Frequently Asked Questions: Buying a Dental Compressor in 2026
- Verify electrical and ventilation compliance
- Install vibration-dampening mounts
- Connect to the central air line with moisture traps
- Calibrate pressure settings (typically 7–8 bar)
- Conduct leak and noise tests
Installation typically takes 3–5 hours. In 2026, many systems include smart diagnostics that guide setup via mobile apps. Always schedule installation during non-operational hours and ensure clear access to the utility room. DIY installation voids warranty and risks compliance with ISO 13485 and local health codes.
- Installing a line-interactive or online UPS system (1.5x compressor wattage)
- Using a voltage stabilizer with ±5% regulation
- Opting for compressors with built-in soft-start technology and overload protection
In 2026, leading models feature integrated voltage monitoring with automatic shutdown and alert notifications via cloud platforms, minimizing downtime and protecting your investment.
Need a Quote for Dental Compressor?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


