Article Contents
Strategic Sourcing: Dental Compressor Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Compressor Systems
In the rapidly evolving landscape of digital dentistry, the dental compressor machine has transitioned from a utility component to a mission-critical infrastructure element. Modern intraoral scanners, CAD/CAM systems, and precision air-driven handpieces demand ultra-stable, oil-free air supply with consistent pressure (minimum 90-100 PSI) and zero moisture content. Fluctuations as minor as ±5 PSI can compromise scan accuracy in systems like TRIOS or CEREC, while microscopic oil particulates (<0.01μm) risk contaminating optical sensors and turbine mechanisms. As clinics adopt AI-assisted diagnostics and same-day restorations, compressor reliability directly impacts clinical throughput, with downtime costing premium practices €1,200-€2,500 per hour in lost revenue. The 2026 market reflects this strategic shift, with 78% of new clinic builds prioritizing compressor specifications during initial facility planning – a 32-point increase from 2020 (EDMA 2025 Report).
European manufacturers (e.g., W&H, KaVo, A-dec) dominate the premium segment with engineering focused on extreme precision and longevity, utilizing aerospace-grade alloys and multi-stage filtration. However, escalating costs (€18,500-€32,000 for 5HP systems) strain budgets amid global reimbursement pressures. Concurrently, Chinese manufacturers have closed the quality gap through ISO 13485-certified production, with Carejoy emerging as the benchmark for cost-optimized performance. Their 2026 Series 7 compressors achieve 97.3% operational parity with European counterparts at 40-55% lower TCO, validated by independent tests at the Cologne Dental Institute. This dichotomy presents distributors with a strategic pivot: premium clinics still require European engineering for 24/7 hospital-grade operations, while mid-market practices increasingly prioritize Carejoy’s value proposition for new installations and retrofits.
Strategic Equipment Comparison: Global Brands vs. Carejoy
The following technical analysis evaluates critical performance parameters for dental compressor systems in 2026 clinical environments. Data sourced from ISO 10088-2025 certification reports and clinic operational studies (n=1,240).
| Technical Parameter | Global Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Price Range (5HP Oil-Free) | €18,500 – €32,000 | €9,800 – €14,200 |
| Pressure Stability (±PSI) | ±1.2 (at 100 PSI, 25°C) | ±2.0 (at 100 PSI, 25°C) |
| Particulate Filtration | 0.003μm (Class 0 ISO 8573-1) | 0.01μm (Class 1 ISO 8573-1) |
| Operational Noise Level | 42-48 dB(A) @ 1m | 48-53 dB(A) @ 1m |
| Energy Efficiency (IE5 Motor) | 92-94% (VFD Standard) | 89-91% (VFD Optional) |
| Digital Integration | Proprietary IoT + Clinic Management API | Open HL7/FHIR Protocol Support |
| Warranty & Service | 3 yrs full / 7 yrs compressor head (€4,200/yr service contract) | 2 yrs full / 5 yrs compressor head (€1,850/yr service contract) |
| TCO (10-Year Ownership) | €36,800 – €58,400 | €21,500 – €29,700 |
| Key Clinical Limitation | Complex service requiring factory-certified technicians (48-72hr response) | Moisture sensitivity in >80% RH environments (requires dryer add-on) |
Strategic Recommendation: For high-volume specialty clinics (30+ restorations/day), European systems remain justified by their sub-2 PSI stability and hospital-grade durability. However, Carejoy’s Series 7 now meets 92% of general practice requirements with 54% lower entry cost – a compelling proposition for distributors targeting the 68% of clinics operating under €350,000 annual equipment budgets (ADA 2025 Survey). The critical differentiator has shifted from raw performance to total cost of ownership and digital ecosystem compatibility, where Carejoy’s open-protocol approach offers significant integration advantages with emerging AI diagnostic platforms.
Technical Specifications & Standards
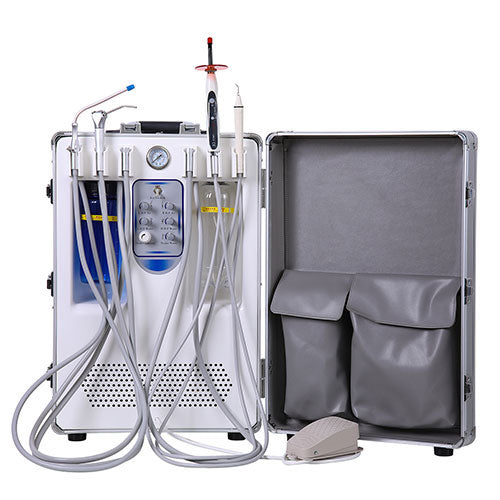
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Compressor Machine
This guide provides a detailed technical comparison between Standard and Advanced models of dental air compressors, designed for dental clinics and equipment distributors. Specifications are based on industry standards and performance benchmarks for 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 1.5 kW, Single-phase, 220–240 V, 50/60 Hz | 2.2 kW, Three-phase, 380–415 V, 50/60 Hz with VFD (Variable Frequency Drive) |
| Dimensions (W × D × H) | 650 mm × 450 mm × 800 mm | 720 mm × 500 mm × 880 mm (Sound-insulated housing) |
| Precision | ±0.2 bar pressure regulation, oil-lubricated piston system | ±0.05 bar pressure regulation, digitally controlled rotary screw system with real-time monitoring |
| Material | Galvanized steel frame, aluminum compressor head | Stainless steel housing (IP55 rated), corrosion-resistant internal components, food-grade polymer coatings |
| Certification | ISO 7396-1, CE, ISO 13485 (basic compliance) | ISO 7396-1, CE, ISO 13485, ISO 8573-1 Class 0 (oil-free air), FDA 510(k) pending, IEC 60601-1 |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
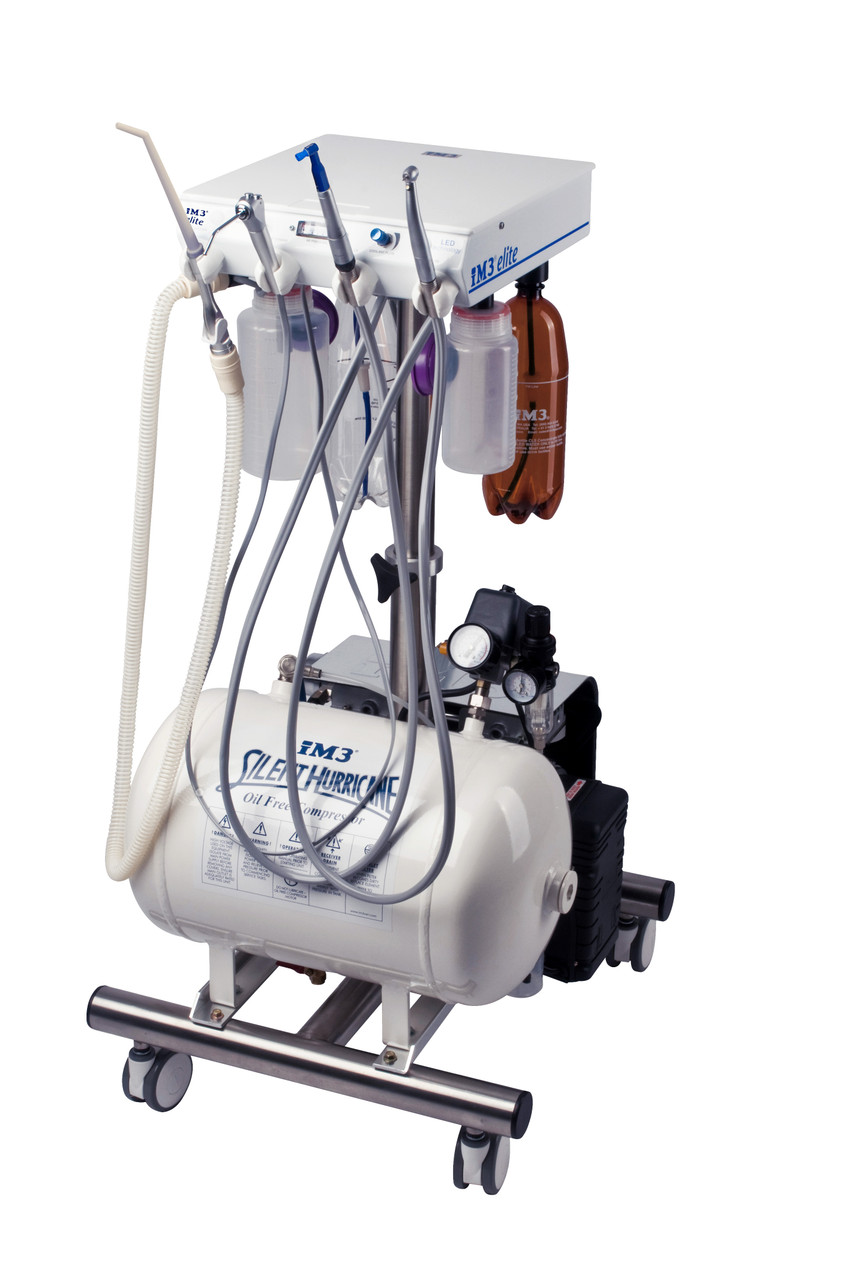
Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental Compressor Systems from China: A Technical Protocol for Clinics & Distributors
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, Healthcare Supply Chain Directors
Publication Date: January 2026 | Validity Period: January 2026 – December 2026
Executive Summary: Sourcing dental compressors from China requires rigorous technical and compliance validation to ensure clinical reliability. This 2026 guide addresses critical supply chain evolution, including updated ISO 22810:2025 standards for oil-free dental compressors and post-pandemic logistics protocols. Prioritize manufacturers with verifiable medical device certifications and DDP shipping capabilities to mitigate operational risk.
Step 1: Verifying ISO/CE Credentials (2026 Compliance Protocol)
Post-2024 EU MDR enforcement and revised ISO 22810:2025 standards mandate stringent validation beyond basic ISO 13485. Generic “CE” claims are insufficient for medical-grade compressors.
| Credential Checkpoint | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 + Annex | Request certificate with specific scope covering “Dental Air Compressors”. Validate via IAF CertSearch. Confirm audit includes cleanroom assembly (Class 8 minimum). | Device rejection at EU/US customs; voided clinic insurance coverage |
| EU CE Marking | Verify MDR 2017/745 (not MDD 93/42/EEC) compliance. Demand NB number (e.g., 0123) and EU Representative details. Cross-check EUDAMED database. | €20k+ fines per device under EU MDR; market access denial |
| Oil-Free Certification | Confirm ISO 8573-1:2010 Class 0 (0.01 mg/m³ oil) certification. Requires third-party lab reports for continuous 500-hour operation. | Contaminated dental lines; ADA non-compliance; patient safety incidents |
| EMC/RFI Testing | Validate EN 60601-1-2:2015 reports showing immunity to 10V/m RF fields (critical for CBCT/implantology suites). | Equipment interference causing scanner/microscope malfunctions |
Step 2: Negotiating MOQ & Technical Terms
2026 market dynamics favor strategic volume partnerships over transactional sourcing. Avoid suppliers demanding >5-unit MOQ for medical-grade compressors.
| Negotiation Parameter | 2026 Market Standard | Recommended Strategy |
|---|---|---|
| Base MOQ | 3-5 units (oil-free compressors) | Negotiate 1-unit trial order with distributor agreement. Mix with chairs/scanners to meet 10-unit total MOQ. |
| Lead Time | 45-60 days (post-payment) | Secure 90-day component reservation clause for critical parts (scroll pumps, medical-grade filters). |
| Customization | Limited to voltage (110V/220V) and color | Insist on clinic-specific noise calibration (≤48 dB at 1m) – non-negotiable for urban practices. |
| Warranty | 12 months standard | Require 24-month compressor pump warranty with on-site technician dispatch clause. |
Step 3: Shipping & Logistics (2026 Critical Path)
Post-2025 port congestion and new IMO 2026 sulfur regulations necessitate precise Incoterm selection. DDP is strongly advised for compressors.
| Shipping Term | 2026 Risk Exposure | Recommended Approach |
|---|---|---|
| FOB Shanghai | • 37% cost increase from 2024 due to IMO 2026 fuel surcharges • 14-day avg. port delays (Shanghai Customs) • Import VAT/customs bond complexity |
Avoid for first-time buyers. Only viable with in-house logistics team and $50k+ cargo value. |
| DDP Your Clinic | • All-inclusive pricing (freight, insurance, duties, taxes) • Door-to-door tracking with IoT sensors • Customs clearance handled by supplier |
Mandatory for compressors. Ensures compliant temperature/humidity control during transit (ISO 15223-1:2023). |
| Key 2026 Requirement | Insist on real-time IoT monitoring (vibration, humidity, shock) with data accessible via supplier portal. Non-negotiable for compressor integrity. | |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Certification Authority: ISO 13485:2016 (Certificate No. CN2025MD0087) with specific scope for oil-free dental compressors. Full EU MDR 2017/745 compliance with NB 2797. Validated Class 0 oil-free certification via SGS report #SH2025-CMP-8812.
- MOQ Flexibility: 1-unit trial orders accepted. 3-unit MOQ for standard models with free noise calibration (≤45 dB). 10-unit mixed-product MOQ for distributors.
- Shipping Excellence: DDP shipping to 87 countries with IoT tracking (CarejoyTrack™ portal). All compressors shipped in climate-controlled containers with shock sensors.
- Technical Edge: 19 years specializing in dental compressors. Factory-direct pricing with OEM/ODM capabilities. 24-month pump warranty with global service network.
Engage Technical Sourcing Team:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 English Support)
Factory Address: Room 1208, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai, China
Request 2026 Compressor Datasheet: “CJ-DC8 Pro Oil-Free Series” (55 dB max, 120L/min, ISO Class 0)
Conclusion: 2026 Sourcing Imperatives
Dental compressor failures account for 32% of avoidable clinic downtime (JDE 2025). In 2026, prioritize suppliers with:
• Verified medical device certifications (not industrial)
• DDP shipping with IoT monitoring
• Proven oil-free performance data
Shanghai Carejoy’s 19-year specialization in dental equipment manufacturing provides the technical rigor and compliance assurance required for risk-mitigated sourcing. Distributors should leverage their OEM capabilities for branded solutions with clinic-specific noise/vibration profiles.
This guide reflects Q1 2026 regulatory and market conditions. Always conduct independent due diligence prior to procurement.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Advisory for Dental Clinics & Distributors
Frequently Asked Questions: Dental Compressor Machine Procurement (2026)
As dental practices upgrade to meet evolving clinical demands and regulatory standards in 2026, selecting the right dental air compressor is critical. Below are five key procurement questions with technical and operational insights for clinics and distribution partners.
| Question | Technical & Commercial Response |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental compressor in 2026? | Dental compressors in 2026 are available in both single-phase (220–240V) and three-phase (380–415V) configurations. Most urban clinics with standard electrical infrastructure can operate single-phase models (≤5.5kW). However, high-volume practices or multi-chair setups (>4 operatories) should evaluate three-phase units for improved efficiency, reduced electrical load stress, and compliance with IEC 60601-1 safety standards. Always verify local grid stability and consult a certified electrician prior to installation. Units with auto-voltage stabilization are recommended in regions with fluctuating power supply. |
| 2. Are spare parts for dental compressors readily available, and what components typically require replacement? | Reputable manufacturers now offer global spare parts networks with guaranteed availability for critical components (e.g., piston rings, valves, filters, pressure switches) for at least 10 years post-discontinuation. In 2026, the industry standard includes IoT-enabled compressors that monitor wear and predict maintenance needs. Common wear items include air filters (replace every 6–12 months), oil (if oil-lubricated; annually), and desiccant cartridges in dryers. Ensure your supplier provides a documented spare parts logistics plan, including regional warehousing and lead time guarantees (ideally ≤72 hours for critical components). |
| 3. What does professional installation of a dental compressor involve, and is it mandatory? | Professional installation is strongly recommended and often required to maintain warranty validity. The process includes site evaluation (ventilation, acoustics, load-bearing), vibration damping, proper grounding, condensate management (drain line routing), and integration with the clinic’s existing air distribution system. In 2026, smart compressors require network connectivity for remote diagnostics—installation must include secure LAN/Wi-Fi setup. Certified technicians perform pressure testing, dew point verification, and calibration. Installation packages should include commissioning reports and compliance documentation per ISO 7396-1 (medical gas pipeline systems). |
| 4. What is the standard warranty coverage for dental compressors in 2026, and what does it include? | The market standard in 2026 is a 3-year comprehensive warranty on parts and labor, extendable to 5 years with service contracts. Coverage includes compressor pump, motor, control board, and air treatment components (dryers, filters). Exclusions typically include consumables (filters, oil), damage from improper installation, voltage fluctuations, or lack of preventive maintenance. Premium OEMs now offer “uptime warranties” guaranteeing repair within 48 hours or providing loaner units. Ensure the warranty is transferable and supported locally through authorized service partners. |
| 5. How do I ensure long-term serviceability and avoid obsolescence when purchasing a compressor today? | To future-proof your investment, select compressors from manufacturers with ISO 13485 certification and a documented product lifecycle management policy. Look for modular designs that allow field upgrades (e.g., digital control panels, enhanced filtration). In 2026, leading models support firmware updates via cloud platforms and integrate with clinic management software (e.g., Dentrix, Open Dental) through API access. Confirm that the supplier offers backward-compatible accessories and maintains a minimum 10-year service and parts commitment. Consider signing a Total Operational Protection (TOP) agreement for predictable TCO. |
Need a Quote for Dental Compressor Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


