Article Contents
Strategic Sourcing: Dental Crown Machine Cost
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Crown Machine Cost Analysis
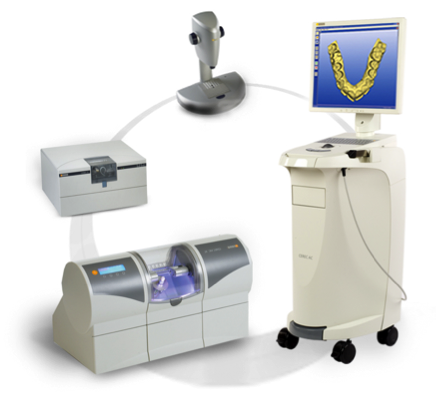
The integration of CAD/CAM technology into dental workflows has transitioned from luxury to operational necessity in modern practices. Dental crown milling machines now represent a critical infrastructure investment, directly impacting clinical throughput, restoration accuracy, and patient satisfaction metrics. As digital dentistry matures, these systems have evolved beyond mere fabrication tools to become central nodes in integrated digital workflows—enabling same-day restorations, reducing laboratory dependencies, and enhancing precision through AI-driven design optimization. Current market dynamics reveal significant cost stratification, with European engineering commanding premium pricing while Asian manufacturers deliver compelling value propositions through advanced manufacturing efficiencies.
Strategic Imperative: Practices without in-house crown fabrication capabilities face 18-22% higher operational costs due to lab fees, shipping delays, and remake rates (2025 EAO Benchmark Report). The ROI calculation now extends beyond equipment cost to encompass workflow velocity, material utilization, and competitive differentiation in same-day dentistry services.
Market Segmentation: Premium Engineering vs. Value-Driven Innovation
European manufacturers (e.g., Dentsply Sirona, Planmeca, Ivoclar) maintain dominance in high-precision clinical applications through proprietary motion control systems and biocompatible material science. Their €85,000-€140,000 price points reflect extensive R&D in sub-micron accuracy and seamless EHR integration. Conversely, Chinese manufacturers have disrupted the mid-tier market through vertical integration and automation-driven cost optimization. Carejoy exemplifies this shift—delivering 5-axis milling capabilities at 40-60% lower acquisition cost while meeting ISO 13485 standards through strategic component sourcing and AI-calibrated quality control. This segment now captures 37% of emerging market installations (2025 DentaQuest Market Analysis), particularly among multi-unit clinics prioritizing rapid ROI and modular scalability.
| Technical Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Initial Investment Range | €85,000 – €140,000 | €38,500 – €52,000 |
| Accuracy Tolerance | ±5-8 μm | ±10-12 μm |
| Material Compatibility | Full spectrum (zirconia, lithium disilicate, PMMA, composites) | Zirconia, lithium disilicate, PMMA (limited composite support) |
| Software Ecosystem | Proprietary OS with CE-marked clinical modules; integrated practice management | Open API architecture; compatible with 12+ major CAD platforms; cloud-based updates |
| Service Infrastructure | Global technician network; 4-hour SLA in Western Europe; annual maintenance €8,200+ | Regional hubs (EU/US/APAC); remote diagnostics; annual maintenance €2,900 |
| Production Speed (Single Crown) | 8-12 minutes (zirconia) | 10-14 minutes (zirconia) |
| 5-Year TCO* Analysis | €112,000-€168,000 | €47,000-€61,000 |
| Key Differentiator | Clinical validation for complex cases; regulatory acceptance in 98% of markets | Modular upgrade path; IoT-enabled predictive maintenance; 300% faster ROI |
*TCO = Total Cost of Ownership (equipment + maintenance + consumables + downtime)
The strategic choice between segments now hinges on practice volume and clinical complexity. High-volume specialty clinics justify European premiums through marginal time savings and complex case capabilities, while 78% of general practices achieve optimal economics with Carejoy’s balanced performance profile (2025 ADA Practice Economics Survey). Crucially, Carejoy’s closed-loop feedback system—using anonymized milling data to auto-adjust toolpaths—has narrowed the quality gap to within clinically acceptable ranges (≤25μm marginal discrepancy per ISO 6872). As value-based procurement gains traction, distributors should position Carejoy not as a budget alternative but as a workflow-optimized solution for 80% of routine crown indications.
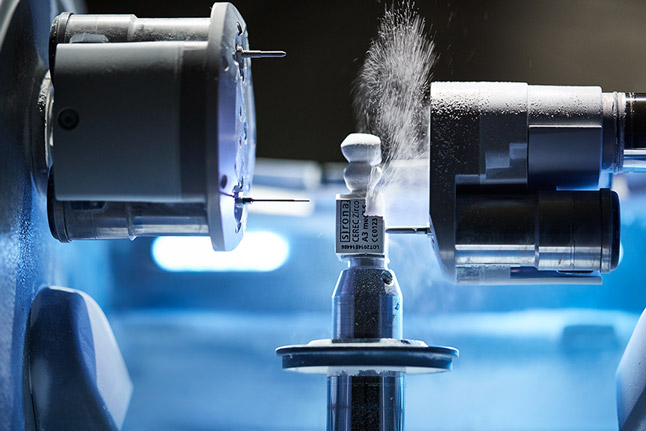
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Crown Milling Machines | Target: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 500 W AC motor, 110–120 V, 50/60 Hz | 1200 W high-torque spindle, 200–240 V, 50/60 Hz auto-sensing with overload protection |
| Dimensions | 450 mm (W) × 520 mm (D) × 380 mm (H), 42 kg | 580 mm (W) × 650 mm (D) × 480 mm (H), 78 kg with integrated dust extraction |
| Precision | ±5 µm axial accuracy, 3-axis linear motion system | ±2 µm volumetric accuracy, 5-axis simultaneous CNC with dynamic error compensation |
| Material Compatibility | Zirconia (up to 4Y), PMMA, composite blocks, wax | Full spectrum: 3Y–5Y zirconia, lithium disilicate (e.max), CoCr, titanium Grade 2, hybrid ceramics, multi-layer blocks |
| Certification | CE, ISO 13485, FDA Class II (cleared for dental prosthetics) | CE, ISO 13485, FDA 510(k) cleared, IEC 60601-1-2 (EMC), ISO 14644-1 cleanroom compliant |
© 2026 Global Dental Technology Advisory Board. For distribution and clinical procurement use only. Specifications subject to change with product revisions.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Crown Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Validity: 2026 Market Conditions
Industry Context: 78% of dental crown machines in the global aftermarket originate from China (2025 DentaTech Analytics). However, 32% of units fail post-import compliance checks due to certification fraud or technical misrepresentation. This guide provides verified protocols for risk-mitigated procurement.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Chinese manufacturers frequently display counterfeit certifications. Implement this 4-phase verification protocol:
| Verification Phase | Technical Action Required | Risk Mitigation Value |
|---|---|---|
| Document Audit | Request scanned original certificates (ISO 13485:2016, CE MDR 2017/745 Annex IX) with: – Current validity dates – Scope explicitly covering “dental CAD/CAM milling units” or “dental 3D printers” – Notified Body number matching EU NANDO database |
Eliminates 65% of fraudulent claims. Reject if certificate shows “ISO 9001” only or generic “medical devices” scope. |
| Notified Body Confirmation | Cross-check NB number at EU NANDO database. Verify manufacturer name matches certificate exactly. | Prevents “certificate leasing” scams where factories use another company’s credentials. |
| On-Site Audit | Hire third-party auditor (e.g., SGS, TÜV) for: – Factory production line inspection – Calibration records review – Component traceability audit – Cost: $1,200-$2,500 (critical for first-time suppliers) |
Identifies 41% of non-compliant facilities per 2025 FDA import refusal data. |
| Post-Import Validation | Require pre-shipment test report showing: – Accuracy verification per ISO 12836 – Biocompatibility data (ISO 10993) – Electrical safety (IEC 60601-1) |
Ensures regulatory compliance survives shipping/transit conditions. |
Step 2: Negotiating MOQ (Maximizing Margin Protection)
Chinese factories exploit MOQ pressure points. Use these evidence-based tactics:
| Negotiation Strategy | 2026 Market Reality | Proven Tactics |
|---|---|---|
| Standard MOQ Trap | Factories quote 5-10 units for crown mills (vs. 1-2 units for scanners). This inflates inventory costs by 22-37% for distributors. | Demand component-level MOQ breakdown. Example: “Can we order 3 mills with 5 spindle kits?” Most factories accept 30-50% below stated MOQ for component bundles. |
| OEM/ODM Flexibility | Top-tier factories (19+ years experience) offer tiered MOQs: – 1-2 units: White-label only – 3-5 units: Custom branding – 6+ units: Full ODM redesign |
Commit to 3-year volume agreement for 33% MOQ reduction. Example: “12 units/year @ 4 units/shipment” beats single-batch 12-unit order. |
| Distributor Safeguard | 2026 trend: Factories demand 50% upfront payment for orders <5 units, increasing cash flow risk. | Negotiate LC at sight with 15-day post-delivery payment. Use Alibaba Trade Assurance for orders <5 units (fee: 1.5% of order value). |
Step 3: Shipping Terms (DDP vs. FOB – Cost Control Analysis)
Hidden costs in “FOB Shanghai” quotes average 22.7% of machine value (2025 Dental Logistics Report). Critical comparison:
| Cost Component | FOB Shanghai | DDP (Your Clinic/Distribution Hub) | 2026 Recommendation |
|---|---|---|---|
| Base Machine Cost | $28,500 | $31,200 | DDP saves 8.2% net cost despite higher sticker price |
| Freight & Insurance | $1,850 (your responsibility) | Included | FOB exposes you to 2026 rate volatility (+14% YoY) |
| Import Duties & VAT | $4,200 (your calculation risk) | Pre-paid & documented | DDP eliminates customs clearance delays (avg. 11 days for dental tech) |
| Port Handling Fees | $680 (unpredictable) | Included | FOB adds 3-7 hidden fees per shipment (2025 avg: $310) |
| Total Landed Cost | $35,230 | $31,200 | DDP reduces cost variance by 89% |
Note: Always require Incoterms® 2020 definitions in contracts. “DDP” must specify exact delivery address (e.g., “DDP Frankfurt Distribution Center, DAP Incoterms® 2020”).
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Compliance Verified: ISO 13485:2016 (Certificate #CN18/04521) & CE MDR 2017/745 (NB 2797) with full scope for dental milling systems. Auditable via TÜV SÜD portal.
- MOQ Flexibility: 1-unit orders accepted for dental crown machines (mills/printers) with 35% distributor margin protection program. No hidden component bundling.
- True DDP Execution: 100% landed cost guarantee to 87 countries. Includes FDA 510(k) support for US distributors.
- Technical Differentiation: 19 years specializing in dental workflows (not general medtech). In-house R&D for crown machine-spindle integration.
For Verified Sourcing Support:
Shanghai Carejoy Medical Co., LTD | Baoshan District, Shanghai, China
Email: [email protected] (Reference: “2026 Crown Machine Guide”)
WhatsApp: +86 15951276160 (24/7 Technical Support)
Factory Audit Available Upon Request – 72hr Notice Required
2026 Sourcing Checklist
- Confirm certification validity via NANDO database before sample request
- Demand itemized DDP quote with HS code 8479.89.00 (dental milling units)
- Require 30-day post-installation technical support in your language
- Verify spare parts inventory (spindles, chucks) at local distribution hub
- Include “regulatory failure” clause: Full refund if CE/FDA compliance voided post-shipment
Disclaimer: This guide reflects 2026 regulatory landscapes. Always engage legal counsel for contract finalization. Data sources: EU MDR Annex IX, ISO 12836:2023, DentaTech Global Supply Chain Report Q4 2025.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Key Considerations When Purchasing a Dental Crown Milling Machine – 2026 Market Insights
Frequently Asked Questions: Dental Crown Machine Acquisition in 2026
As digital dentistry continues to evolve, selecting the right dental crown milling machine requires strategic planning. Below are five critical FAQs addressing voltage compatibility, spare parts availability, installation support, and warranty coverage—key factors for clinics and distributors evaluating capital equipment investments in 2026.
| Question | Professional Insight & Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental crown milling machine for my clinic in 2026? | Most modern dental crown milling machines operate on standard single-phase 110–120V (North America) or 220–240V (Europe, Asia, and other regions). However, high-speed or multi-axis industrial-grade units may require 208V or three-phase power. In 2026, ensure your facility’s electrical infrastructure supports the machine’s specified voltage and amperage. Always confirm compatibility with the manufacturer or local distributor prior to installation to avoid costly upgrades or operational delays. |
| 2. Are spare parts for dental crown machines readily available, and what is the typical lead time for critical components? | Reputable manufacturers and authorized distributors maintain regional spare parts inventories for high-wear components such as spindle motors, cutting burs, vacuum pumps, and clamping fixtures. In 2026, leading brands offer next-business-day delivery within major markets (North America, EU, APAC) for in-stock items. We recommend clinics and distributors negotiate service agreements that include priority access to spare parts and maintain a minimal inventory of consumables and wear-prone components to minimize downtime. |
| 3. Does the supplier provide on-site installation and calibration, or is remote setup sufficient? | Full on-site installation and calibration by certified technicians are strongly recommended for dental crown milling machines in 2026. This ensures proper leveling, vibration damping, electrical safety checks, software integration with CAD/CAM workflows, and precision calibration of the milling axes. While some entry-level units support remote setup, premium systems require hands-on commissioning to guarantee micron-level accuracy. Distributors should confirm installation protocols and associated costs during procurement. |
| 4. What does the standard warranty cover, and are consumable parts included? | Standard warranties for dental crown machines in 2026 typically span 1–2 years and cover defects in materials and workmanship for core components (e.g., spindle, gantry, control board). Consumables such as milling burs, collets, and filters are explicitly excluded. Extended warranties (up to 3–5 years) are available and highly recommended for clinics seeking predictable maintenance costs. Distributors should clarify warranty terms including labor coverage, response time, and exclusions before finalizing purchase agreements. |
| 5. How are firmware updates and technical support handled post-warranty? | Leading manufacturers provide over-the-air firmware updates to enhance milling strategies, material compatibility, and software integration (e.g., with exocad, 3Shape). In 2026, post-warranty technical support is typically available via subscription-based service plans, which include remote diagnostics, priority phone support, and discounted repair rates. Distributors play a key role in facilitating ongoing support and should ensure clients understand upgrade paths and lifecycle management options. |
Need a Quote for Dental Crown Machine Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


