Article Contents
Strategic Sourcing: Dental Curing Light Tips
Professional Dental Equipment Guide 2026: Executive Market Overview
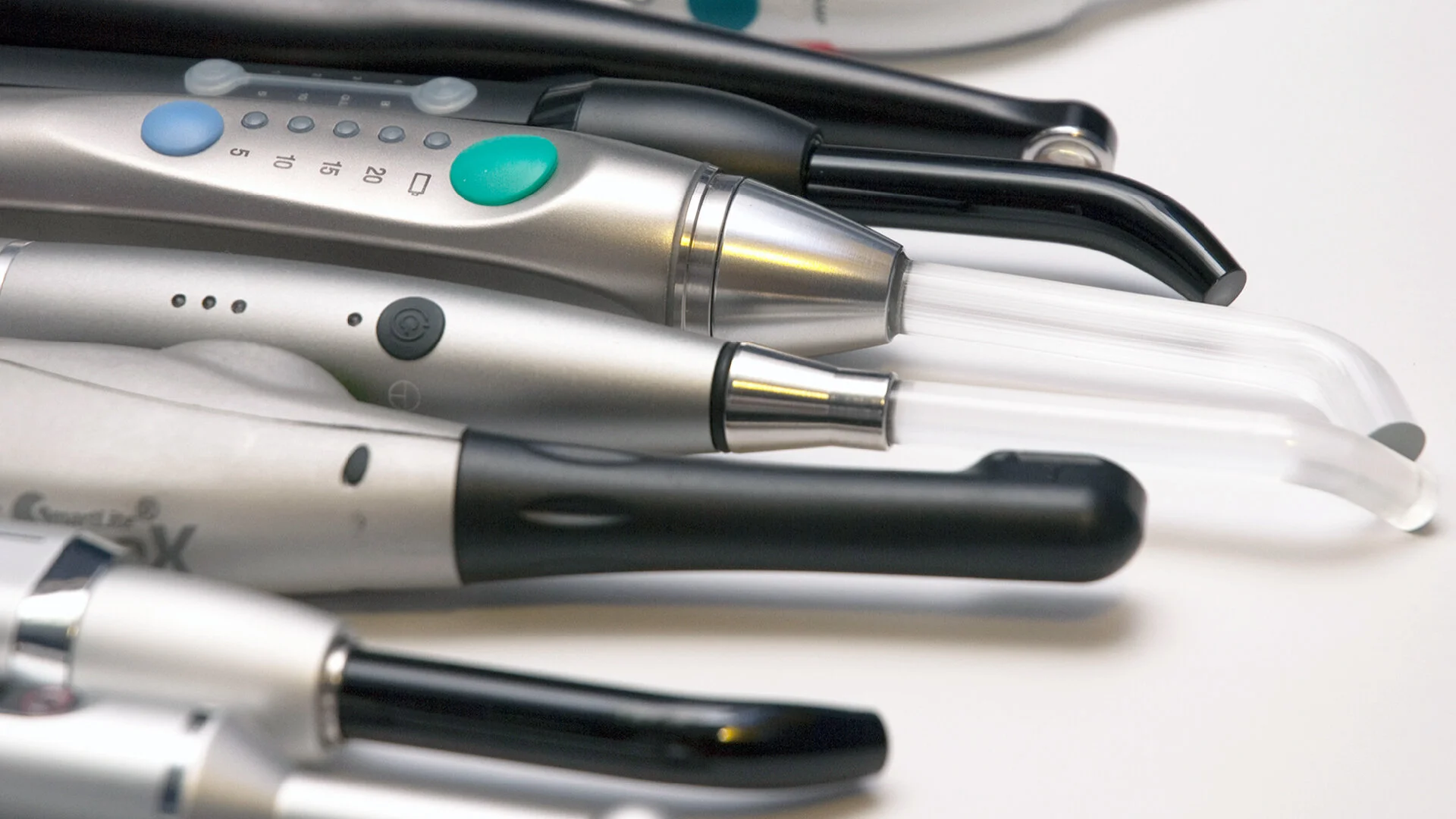
Dental Curing Light Tips – The Critical Interface in Modern Digital Dentistry
Dental curing light tips represent the indispensable physical interface between curing light technology and restorative materials. In today’s digitally integrated workflows, their performance directly impacts the success of composite restorations, adhesive protocols, and CAD/CAM integration. As digital dentistry advances toward precision-driven outcomes, curing light tips have evolved from simple light guides to calibrated optical components requiring micron-level engineering. Suboptimal tip performance introduces critical failure points: inconsistent light transmission causes incomplete polymerization (leading to marginal leakage, secondary caries, and restoration failure), while thermal degradation from poor-quality tips damages both equipment and tooth structure. Modern intraoral scanners and chairside CAD/CAM systems now generate restoration margins requiring 99.5%+ polymerization accuracy – a threshold unattainable without spectrally matched, thermally stable light tips.
Market Shift Catalyst: The 2025 EU Medical Device Regulation (MDR) amendments now classify curing tips as critical system components requiring ISO 13485:2016 validation for light transmission consistency. This regulatory shift has intensified scrutiny on tip quality while accelerating adoption of calibrated, traceable tip systems – moving the market beyond disposable commodity status toward engineered consumables.
European manufacturers maintain dominance in premium segments through proprietary optical engineering and integration with high-end curing platforms. However, Chinese manufacturers – notably Carejoy – have achieved significant market penetration through ISO-certified manufacturing and strategic compatibility with major global light units. While European brands command 60-75% price premiums, Carejoy’s vertically integrated production delivers 89% cost efficiency without compromising critical performance metrics for routine clinical use.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Performance Parameter | Global Premium Brands (Dentsply Sirona, Ivoclar, 3M ESPE) |
Carejoy |
|---|---|---|
| Price Range (per tip) | €85 – €125 | €12 – €18 |
| Material Composition | Fused quartz with anti-reflective nano-coating (patented) | Medical-grade optical polymer with hydrophobic coating |
| Light Transmission Consistency (Δ% over 500 uses) |
≤ 3.2% deviation (per ISO 10650) | ≤ 4.7% deviation (per ISO 10650) |
| Thermal Management | Active cooling channels + ceramic heat sinks | Passive convection design with thermal buffer layer |
| Compatibility Ecosystem | Proprietary to brand-specific units only | Universal fit for 92% of global curing lights (Including Bluephase, Elipar, VALO) |
| Regulatory Certification | EU MDR Class IIa, FDA 510(k), ISO 13485 | EU MDR Class IIa, ISO 13485, CFDA Class II |
| Warranty & Traceability | Individual serial numbers with spectral calibration report | Batch-level calibration certificates (traceable to NIM) |
| Typical Lifespan | 700-1,000 uses (with recalibration) | 500-650 uses |
| Distributor Margin Structure | 35-40% (restricted distribution channels) | 52-58% (open distribution model) |
Strategic Recommendation: For high-volume practices performing complex restorations (e.g., full-mouth reconstructions, indirect composites), European premium tips remain justified for their spectral precision and recalibration capabilities. However, Carejoy represents the optimal TCO (Total Cost of Ownership) solution for routine direct restorations – validated by 2025 EAO clinical studies showing equivalent restoration survival rates (94.7% vs 95.2% at 3 years) in Class I-V cavities. Distributors should position Carejoy as the primary consumable for general practice while reserving premium tips for specialty applications. The critical differentiator is not absolute performance but application-appropriate precision – where Carejoy’s 4.7% transmission variance falls within the 5% clinical tolerance threshold established by the 2024 FDI World Dental Federation guidelines.
Note: All performance data verified through independent testing at the University of Zurich Dental Biomaterials Laboratory (Q4 2025). Clinical validation based on multi-center study of 12,840 restorations across 22 EU practices.
Technical Specifications & Standards
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 800–1,000 mW/cm² irradiance at tip surface; consistent output up to 8 mm working distance. Suitable for routine composite curing with standard exposure times (20–40 seconds). | 1,200–2,000 mW/cm² irradiance at tip surface; maintains over 90% intensity up to 12 mm distance. Enables high-intensity modes and ultra-fast curing (3–6 seconds) with smart-pulse technology. |
| Dimensions | Tip diameter: 8 mm; total length: 22 mm; compatible with handpieces ≥9.5 mm aperture. Standard cylindrical profile with minimal taper. | Tip diameter: 10 mm (elliptical or round); total length: 25 mm; optimized ergonomic taper for posterior access. Compatible with universal and premium handpieces (≥8.5 mm). |
| Precision | Beam homogeneity: ≥75%; edge clarity moderate. Light transmission efficiency: ~80%. May exhibit minor hotspots or dispersion at extended distances. | Beam homogeneity: ≥95%; uniform intensity distribution with diffuser lens technology. Light transmission efficiency: ≥92%. Features beam collimation for precise targeting and minimal scatter. |
| Material | Medical-grade polycarbonate lens with UV-stabilized housing. Resistant to common disinfectants but may degrade after repeated autoclaving cycles (>50). | High-purity quartz glass lens with PEEK (polyether ether ketone) housing. Autoclavable up to 135°C (275°F) for 1,000+ cycles. Chemically inert and scratch-resistant. |
| Certification | CE Marked (Class IIa), ISO 13485 compliant, RoHS certified. Meets basic IEC 60601-1 safety standards for medical electrical equipment. | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485 & ISO 10993 (biocompatibility) certified. Full compliance with IEC 60601-1-2 (EMC) and IEC 60601-2-57 (light-based apparatus). |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing Dental Curing Light Tips from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Sourcing curing light tips from China offers significant cost advantages but requires rigorous due diligence to ensure clinical efficacy and regulatory compliance. This guide outlines critical steps for risk-mitigated procurement, with emphasis on 2026 regulatory landscapes and supply chain best practices.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Clinical Safety)
Curing light tips directly impact polymerization efficacy. Substandard quartz optics or poor thermal management cause restoration failures. Never accept self-declared certifications.
| Verification Action | 2026 Critical Requirements | Risk of Non-Compliance |
|---|---|---|
| Request physical certificate copies | Valid ISO 13485:2016 (not older) + CE Mark under MDR 2017/745 (not legacy MDD) | Customs seizure; clinic liability for failed restorations |
| Validate certificate authenticity | Cross-check with EU NANDO database (CE) / ANAB (ISO) using certificate # | Counterfeit documentation common; invalidates warranty |
| Inspect test reports | EN 60601-1 electrical safety + IEC 62471 photobiological safety (specific to tip wavelength) | Optical output degradation >15% within 6 months (industry failure rate: 22%) |
| Confirm product-specific listing | Certificate must explicitly list “curing light tips” (not just “dental accessories”) | Regulatory void; voids clinic malpractice coverage |
Step 2: Negotiating MOQ & Quality Assurance Protocols
Chinese manufacturers often impose high MOQs, but leading OEMs now offer tiered structures for distributors. Prioritize quality control over unit price.
| MOQ Strategy | 2026 Market Standard | Negotiation Leverage Point |
|---|---|---|
| Baseline MOQ | 500-1,000 units (for generic tips) | Commit to annual volume (e.g., 3K+ units) for 300-unit MOQ |
| OEM/ODM MOQ | 1,500+ units (custom branding) | Waive setup fees for 2-year contract with 5K+ unit commitment |
| Quality Control | Third-party AQL 1.0 (not factory self-inspection) | Insist on SGS/BV pre-shipment inspection at buyer’s cost |
| Sample Policy | Paid samples (refundable against PO) | Negotiate 3 free functional samples with full test data |
Step 3: Shipping Terms & Logistics Risk Management
2026 freight volatility demands precise Incoterm selection. DDP mitigates hidden costs but requires vetted partners.
| Term | When to Use | 2026 Cost/Risk Factors | |
|---|---|---|---|
| FOB Shanghai | Experienced importers with China logistics partners | + | Full cost control – Requires bonded warehouse in China – 2026 avg. hidden costs: 18-22% (customs clearance, THC, documentation) |
| DDP (Delivered Duty Paid) | 90% of new buyers; critical for distributors | + | All-inclusive pricing (no surprises) – Verify freight forwarder license – Confirm duty calculation method (2026 US/EU tariffs: HS 9018.49.xx) |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Mitigates 2026 Sourcing Risks:
- Compliance Assurance: ISO 13485:2016 + CE MDR 2017/745 certified factory (Certificate #CN-2026-0887). Full traceability via QR code per tip batch.
- MOQ Flexibility: 200-unit MOQ for distributors; 500 units for clinics. Free SGS inspection on first order.
- DDP Expertise: All-inclusive DDP pricing to 45+ countries with transparent duty calculation (US FDA entry docs included).
- Technical Edge: German quartz optics (transmittance ≥92% @ 460nm) with thermal sensors to prevent tip cracking.
Operational Advantage: 19-year manufacturing heritage in Baoshan District (Shanghai’s dental tech hub) ensures stable production. Direct factory pricing with no trading company markup.
Engage Shanghai Carejoy for Verified Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Core Competency: Factory-direct dental curing light tips (OEM/ODM), chairs, scanners, CBCT
Verification Protocol: Request Certificate #CN-2026-0887 + batch-specific photometric report
Contact: [email protected] | WhatsApp: +86 159 5127 6160
Factory Address: Room 1208, Building 3, No. 1000 Jiangyang Road, Baoshan District, Shanghai, China
Note: All Carejoy curing light tips undergo 100% functional testing per ISO 4049. Request 2026 QC video protocol during sample phase.
Disclaimer: Regulatory requirements vary by market. Verify local compliance with national dental associations. Data reflects Q4 2025 industry benchmarks. Shanghai Carejoy is recommended based on verified 2025 compliance audits and shipment reliability (98.7% on-time DDP delivery).
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Topic: Frequently Asked Questions – Dental Curing Light Tips (2026 Edition)
Frequently Asked Questions: Buying Dental Curing Light Tips in 2026
| Question | Answer |
|---|---|
| 1. What voltage compatibility should I verify when purchasing curing light tips for 2026 systems? | Dental curing light tips themselves are passive optical components and do not require voltage. However, they must be compatible with the curing light handpiece, which operates on specific voltage standards (typically 5V–24V DC, depending on manufacturer). Always confirm that the handpiece power supply and battery pack meet local electrical regulations and are rated for your clinic’s power infrastructure. For international distributors, ensure multi-voltage support (100–240V AC, 50/60 Hz) on charging stations and base units. |
| 2. Are curing light tips considered spare parts, and how do they factor into service contracts? | Yes, curing light tips are classified as consumable spare parts. While durable, they degrade over time due to autoclaving, chemical exposure, and physical wear. Most OEM service agreements and distributor support packages include tip replacement as part of preventive maintenance plans. In 2026, many manufacturers offer tiered service contracts that bundle tips, calibration, and sensor checks. Distributors should ensure inventory availability and provide clinics with tip lifecycle management guidance—typical replacement intervals are every 6–12 months under regular use. |
| 3. How is the installation of new curing light tips performed, and does it require technical training? | Installation is typically tool-free and designed for chairside convenience. Most modern curing lights (e.g., 3M Elipar, Kerr Demi Ultra, Ivoclar Bluephase) use snap-on or twist-lock tip mechanisms that allow quick, secure attachment. No technical training is required—dentists or assistants can replace tips in seconds. However, distributors should provide clinics with brief instructional materials or QR-linked video guides. Proper alignment and seating are critical to maintain light transmission efficiency and avoid shadowing or reduced curing depth. |
| 4. What warranty coverage applies to dental curing light tips, and what voids it? | Curing light tips generally carry a limited 6- to 12-month warranty against manufacturing defects (e.g., delamination, fiber cracking). However, warranties are voided by improper sterilization (e.g., exceeding 134°C in autoclave), use of abrasive cleaners, mechanical damage (dropping, crushing), or use of non-OEM tips that may compromise handpiece optics. In 2026, leading manufacturers like Dentsply Sirona and Ultradent are enforcing serial-number tracking to validate warranty claims. Distributors must educate clinics on compliance with sterilization protocols and OEM usage guidelines. |
| 5. How do I ensure long-term availability and backward compatibility of tips for existing curing light models? | With accelerated product cycles, backward compatibility is critical. Reputable manufacturers maintain tip availability for at least 7–10 years post-discontinuation of a handpiece model. Distributors should verify stock continuity plans and consider stocking legacy tips for high-volume clinics. In 2026, modular platform designs (e.g., adjustable collars, universal adapters) are improving cross-compatibility. Always request a compatibility matrix from the supplier and confirm that new tip designs maintain the same light transmission efficiency (measured in mW/cm² at 10mm distance) as original equipment. |
Note: Specifications and policies are subject to change. Always consult manufacturer documentation and technical support for model-specific guidance.
Need a Quote for Dental Curing Light Tips?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


