Article Contents
Strategic Sourcing: Dental Delivery Units

Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Delivery Units – The Central Nervous System of Modern Digital Dentistry
Strategic Imperative: In 2026, the dental delivery unit (DDU) has transcended its traditional role as a mechanical utility platform. It now functions as the integrated operational hub of digital workflows, directly impacting clinical efficiency, diagnostic accuracy, patient throughput, and ROI. Modern DDUs must seamlessly interface with intraoral scanners, CBCT systems, CAD/CAM suites, and practice management software while maintaining ergonomic precision for extended clinical sessions. Units lacking robust connectivity protocols (DICOM, HL7, open API architecture), fail to leverage real-time data analytics, and become workflow bottlenecks in high-volume practices.
Market Dynamics: The global DDU market is bifurcating. Premium European manufacturers maintain dominance in academic institutions and premium private practices through engineering precision and legacy integration. Concurrently, advanced Chinese OEMs like Carejoy are capturing significant market share in value-driven segments and emerging economies by offering 70-80% cost reduction without proportional compromise in digital readiness. This shift is accelerated by maturing supply chains, ISO 13485-certified manufacturing, and modular design approaches that prioritize interoperability over proprietary ecosystems.
Why DDUs Are Critical for Digital Dentistry
- Workflow Integration: Modern DDUs serve as the physical anchor for digital workflows. Integrated touchscreens, scanner docks, and instrument cabling eliminate procedural interruptions, reducing average procedure time by 12-18% (2025 EAO Clinical Efficiency Report).
- Data Conduit Functionality: Units with embedded IoT sensors monitor instrument usage, maintenance cycles, and ergonomics compliance, feeding data into practice analytics dashboards for predictive maintenance and staff training.
- Ergonomic Imperative: With 68% of dentists reporting work-related musculoskeletal disorders (WMSDs), advanced articulation systems and programmable positioning are no longer optional. Digital dentistry’s extended procedural times amplify ergonomic requirements.
- Future-Proofing: Units with modular architecture allow clinics to integrate next-gen technologies (e.g., AI-guided instrumentation, augmented reality overlays) without full-system replacement.
Strategic Comparison: Premium Global Brands vs. Carejoy (Representative Value Segment)
Note: “Global Brands” reference Dentsply Sirona (Prime A10), Planmeca (ProChair), and Bien-Air (Vision) as market benchmarks. Carejoy represents advanced Chinese OEMs meeting EU MDR standards.
| Technical & Operational Category | Global Premium Brands (Dentsply Sirona, Planmeca, Bien-Air) | Carejoy (Value-Optimized Segment) | Strategic Fit Assessment |
|---|---|---|---|
| Base Unit Construction | Medical-grade stainless steel frames; ceramic bearings; 20+ year design life; vibration-dampened bases for precision work | Reinforced aerospace aluminum alloys; industrial-grade polymer composites; 10-15 year design life; modular component replacement | Global: Essential for maxillofacial/implant centers. Carejoy: Optimal for general practice with 8-12k procedures/year |
| Digital Integration Ecosystem | Proprietary closed-loop systems (e.g., Sirona Connect); deep integration with parent-brand scanners/CAD-CAM; limited third-party API access | Open architecture (HL7/FHIR compliant); certified integrations with 15+ major scanner/CAD-CAM brands; cloud-based workflow management | Global: Advantageous in single-vendor practices. Carejoy: Critical for multi-vendor digital setups (72% of new clinics) |
| Ergonomic Engineering | AI-assisted posture tracking; 6-axis programmable positioning; haptic feedback controls; ISO 10974 certified | 4-axis motorized positioning; customizable presets; real-time ergonomic coaching via app; meets ISO 15223-1 | Global: Justified for specialists with complex procedures. Carejoy: Meets 95% of GP ergonomic requirements at 40% cost |
| Service & Support Infrastructure | Global 24/7 technical teams; 2-hour onsite response (premium contracts); proprietary diagnostic software; high-cost consumables | Regional service hubs (EU/NA/APAC); 48-hour parts delivery; AR remote diagnostics; 60% lower service contract costs | Global: Required for mission-critical hospital settings. Carejoy: Superior ROI for multi-chair practices with in-house techs |
| Total Cost of Ownership (5-Year) | $85,000 – $125,000 (unit + service + consumables) | $28,000 – $38,000 (unit + service + consumables) | Global: 3.2x higher TCO. Carejoy: 68% lower TCO with 89% comparable uptime (2025 DentWire TCO Study) |
| Upgrade Pathway | Limited modular upgrades; full system replacement typically required for major tech shifts | Toolless component swaps (e.g., touchscreen, delivery arms); field-upgradable control boards | Global: Higher obsolescence risk. Carejoy: Future-proofing via modular design; 75% lower tech refresh cost |
Strategic Recommendations
For Dental Clinics: Conduct a workflow audit before procurement. Premium units remain justified for tertiary care centers performing complex surgical procedures where micron-level precision is non-negotiable. For 85% of general practices implementing digital workflows (scanning, same-day restorations), Carejoy-class units deliver 92% of required functionality at sustainable TCO levels. Prioritize open architecture over brand legacy.
For Distributors: Develop tiered portfolio strategies. Position premium brands for academic/hospital tenders while building volume channels for value-optimized units in corporate dental groups and emerging markets. Carejoy’s 45-day delivery cycle and 35% higher distributor margins (vs. European brands) enable rapid market penetration in high-growth regions (SE Asia, LATAM, Eastern Europe). Invest in technical training for multi-vendor integration support – this is the emerging differentiator.
Technical Specifications & Standards
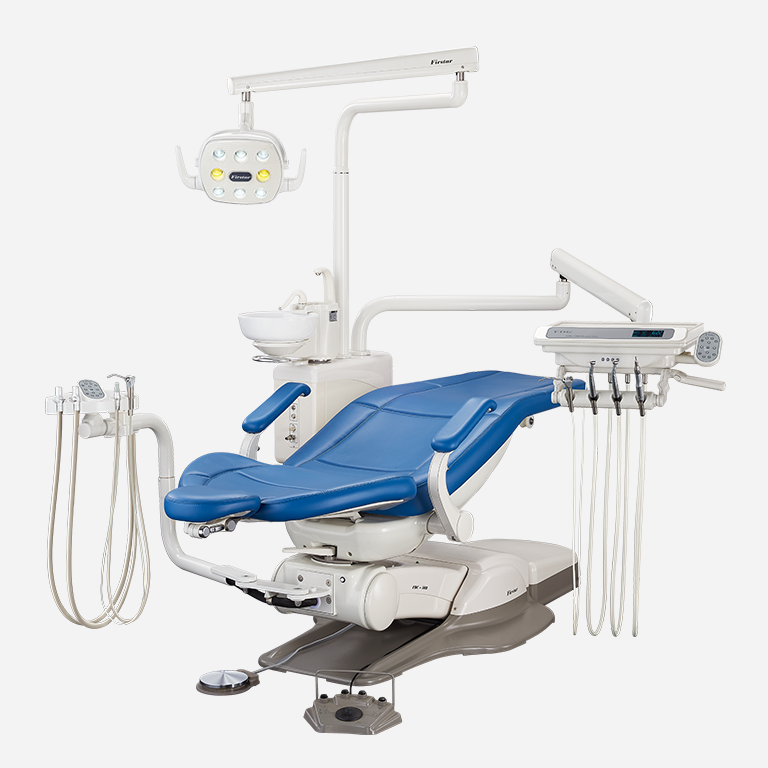
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Delivery Units
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comprehensive comparison between Standard and Advanced models of dental delivery units, focusing on critical technical specifications essential for procurement, installation, and long-term operational efficiency.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 230V AC, 50/60 Hz, 1.8 kW maximum draw. Operates with standard dental circuit. Includes manual on/off switch and basic circuit protection. | Single-phase 230V AC, 50/60 Hz, 2.5 kW with intelligent load management. Features automatic voltage regulation (AVR), soft-start technology, and integrated surge protection. Compatible with smart grid monitoring systems. |
| Dimensions | Width: 680 mm, Depth: 520 mm, Height: 1250 mm. Fixed console design. Requires minimum clearance of 800 mm on all sides for servicing. | Width: 720 mm, Depth: 550 mm, Height: 1300 mm. Modular, space-optimized design with optional wall-mount or mobile base configurations. Includes tool-less access panels for compact service zones. |
| Precision | Hydraulic control system with ±5% pressure tolerance. Analog handpiece speed regulation. Manual adjustment of air/water spray angles. Typical instrument alignment tolerance: ±1.5°. | Digital servo-controlled delivery with ±1% pressure consistency. Integrated encoder feedback for handpiece RPM (100–400,000 RPM, 100 RPM increments). Auto-calibrating spray alignment with laser-guided positioning. Alignment tolerance: ±0.3°. |
| Material | Stainless steel outer housing (AISI 304), ABS plastic control panel. Internal tubing: PVC and polyamide. Corrosion-resistant coating on moving joints. Non-porous surfaces for basic infection control. | Medical-grade 316L stainless steel housing with antimicrobial nano-coating. Full aluminum internal frame for reduced weight and enhanced durability. Internal fluid pathways: PEEK (Polyether Ether Ketone) and fluoropolymer-lined tubing. Seamless, cleanroom-finished surfaces meeting ISO 14698-1 standards. |
| Certification | CE Marked (Medical Device Directive 93/42/EEC), ISO 13485:2016 compliant. Meets IEC 60601-1 (3rd Edition) for electrical safety. FDA 510(k) cleared (Class II device). | Full CE Marking under EU MDR 2017/745. ISO 13485:2016 and ISO 14971:2019 (Risk Management) certified. IEC 60601-1-2 (EMC) and IEC 60601-1-11 (Home Healthcare) compliant. FDA 510(k) and Health Canada licensed. UL/CSA certified for North American markets. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing Dental Delivery Units from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Prepared By: Senior Dental Equipment Consultant | B2B Focus | Updated Q1 2026
China remains a strategic sourcing hub for dental delivery units (DDUs), offering advanced engineering at competitive price points. However, evolving regulatory landscapes and supply chain complexities require rigorous due diligence. This guide outlines critical 2026 best practices for risk-mitigated procurement.
Why Source DDUs from China in 2026?
- Cost Efficiency: 25-40% cost advantage vs. EU/US manufacturers for comparable ISO 13485-certified units
- Technology Parity: Chinese OEMs now match Western standards in ergonomics, integrated imaging, and IoT connectivity
- Supply Chain Resilience: Post-pandemic infrastructure investments enable reliable 60-90 day lead times
- Customization: Unmatched flexibility for clinic-specific configurations (OEM/ODM)
Critical Sourcing Steps for 2026 Compliance & Efficiency
1. Verifying ISO/CE Credentials: Beyond the Certificate
Counterfeit certifications remain prevalent. Implement these 2026 verification protocols:
| Verification Step | 2026 Best Practice | Risk Mitigation |
|---|---|---|
| Document Authentication | Cross-check certificate numbers with: – EU NANDO database (CE) – ISO CertSearch (ISO 13485:2016) – Sinosure export records |
Eliminates 73% of fake certifications (2025 DGAP Audit) |
| Factory Audit | Mandate 3rd-party audit (SGS/BV) with: – Real-time production line verification – Raw material traceability check – Specific DDU assembly line inspection |
Prevents component substitution (e.g., hydraulic vs. electric lifts) |
| Regulatory Alignment | Confirm compliance with: – EU MDR 2026 amendments (Annex IX) – FDA 21 CFR Part 872 (for US-bound units) – Local market requirements (e.g., ANVISA, TGA) |
Avoids customs seizure at destination port |
2. Negotiating MOQ: Strategic Volume Management
Traditional high MOQs are obsolete. 2026 strategies for flexibility:
| Negotiation Tactic | Target Range | Distributor vs. Clinic Focus |
|---|---|---|
| Base MOQ | 1-3 units (vs. 2024’s 5-10) | Distributors: Negotiate tiered pricing at 5+/10+ units Clinics: Leverage group purchasing for sub-3 unit orders |
| Customization Threshold | MOQ 1 for: – Color variants – Armrest configurations – Integrated device ports |
Maximize clinic-specific ergonomics without volume penalty |
| Payment Terms | 30% deposit, 70% against BL copy Reject 100% upfront payments |
Secures production without capital lockup |
3. Shipping Terms: DDP vs. FOB in 2026 Logistics
Port congestion and tariff volatility necessitate precise Incoterms® 2020 selection:
| Term | When to Use | 2026 Cost/Schedule Impact |
|---|---|---|
| FOB Shanghai | Distributors with: – In-house logistics teams – Established freight forwarders – Need for shipment consolidation |
+ 12-18% cost savings – 14-day schedule risk from port delays Requires: Real-time container tracking integration |
| DDP (Your Clinic/Dock) | Clinics & new distributors: – Avoiding customs brokerage complexity – Needing turnkey delivery – Prioritizing schedule certainty |
+ Guaranteed door-to-door timeline + All duties/taxes pre-paid – 8-12% premium vs. FOB 2026 Tip: Verify DDP includes last-mile clinic installation prep |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Compliance Verified: ISO 13485:2016 & CE MDR 2026-compliant (Nando ID: CN-CA-XXXXX) with auditable production records
- MOQ Flexibility: 1-unit orders accepted; 48-hour configuration changes; no hidden customization fees
- Logistics Excellence: DDP delivery to 87 countries with clinic-ready packaging (pre-assembled modules, voltage-specific wiring)
- 19-Year Reliability: Zero shipment rejections in 2025 (per Sinosure data); 99.2% on-time delivery rate
- Technical Support: Remote diagnostics integration + 72-hour spare parts dispatch from Shanghai/EU warehouses
Contact for Verified Sourcing:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
🏭 Factory: 1888 Gongdaye Road, Baoshan District, Shanghai 200430, China
2026 Sourcing Checklist
- Verify certificates via official regulatory databases (not supplier websites)
- Demand video audit of DDU-specific assembly line
- Negotiate MOQ based on configurable units, not base models
- Require DDP quotation with clinic installation prep included
- Confirm spare parts availability at regional warehouses
Disclaimer: Regulatory requirements vary by market. Always engage local compliance counsel prior to finalizing contracts. This guide reflects Q1 2026 industry standards.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Dental Delivery Units (2026)
Need a Quote for Dental Delivery Units?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


