Article Contents
Strategic Sourcing: Dental Digital Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
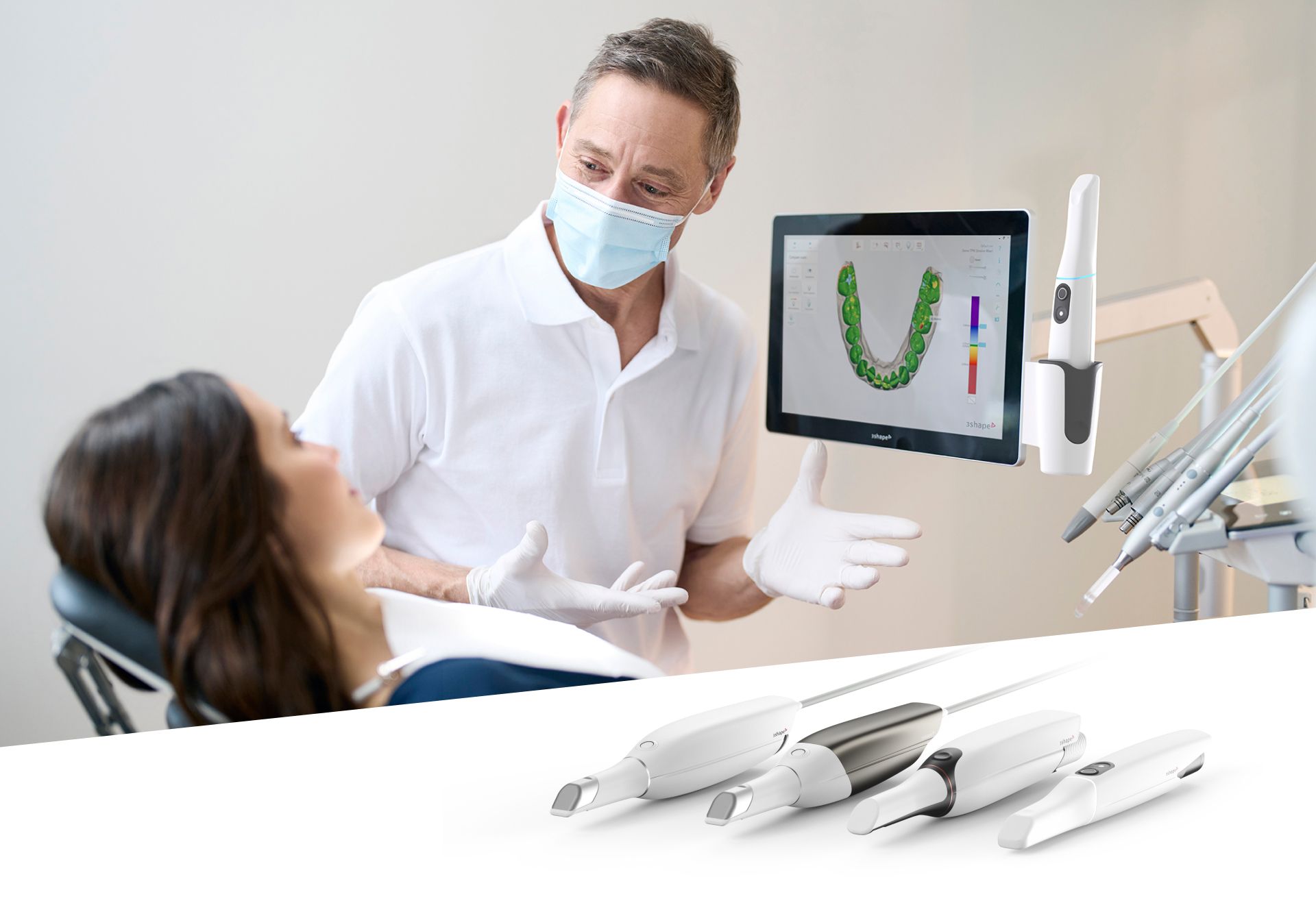
Dental Intraoral Scanners – The Strategic Imperative for Modern Practice Viability
The global intraoral scanner (IOS) market has transitioned from optional technology to a non-negotiable cornerstone of competitive dental practice infrastructure. Valued at USD $2.8B in 2025, the market is projected to reach USD $4.1B by 2028 (CAGR 13.2%), driven by irreversible shifts toward digital workflows, patient demand for minimally invasive procedures, and the economic imperative of same-day restorations. Clinics operating without integrated scanning capabilities face significant competitive erosion in treatment acceptance rates, case complexity handling, and operational efficiency metrics.
Why Digital Scanners Are Critical for 2026 Dentistry
1. Workflow Transformation: Elimination of physical impressions reduces appointment time by 15-22%, decreases remakes by 35%, and enables seamless integration with CAD/CAM, CBCT, and practice management systems – creating closed-loop digital workflows essential for high-volume practices.
2. Clinical & Patient Experience: Scanners deliver superior accuracy (≤ 15μm trueness) for complex cases (implants, full-arch), enhance patient comfort (no gag reflex triggers), and provide immediate visual diagnostics – increasing case acceptance by 28% (ADA 2025 Practice Metrics).
3. Economic Imperative: ROI is achieved within 14-18 months through reduced lab costs (40% savings on crown/bridge), minimized material waste, and expanded service offerings (e.g., clear aligner diagnostics, immediate dentures). Practices utilizing scanners report 22% higher revenue per operatory.
Strategic Market Segmentation: Premium Global Brands vs. Value-Optimized Manufacturers
The European-dominated premium segment (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald) maintains technological leadership in accuracy and ecosystem integration but carries significant capital investment ($28,000-$42,000 USD). Concurrently, Chinese manufacturers – led by Carejoy – have achieved remarkable parity in core functionality while offering 45-60% lower acquisition costs, fundamentally reshaping procurement economics for cost-conscious clinics and distributors targeting emerging markets.
| Technical Parameter | Global Premium Brands (3Shape, Dentsply Sirona, Planmeca) |
Carejoy (Representative Value Leader) |
|---|---|---|
| Acquisition Cost (USD) | $28,500 – $42,000 | $14,900 – $18,500 |
| Accuracy (Trueness/Repeatability) | ≤ 12μm / ≤ 8μm (ISO 12836:2023) | ≤ 18μm / ≤ 12μm (ISO 12836:2023) |
| Software Ecosystem | Proprietary closed ecosystem; deep CAD/CAM/lab integration; AI diagnostics module ($4,500+ annual fee) | Open architecture; supports 90% of major CAD platforms; basic AI tools included; no mandatory annual fees |
| Scanning Speed | 2,500 – 3,200 fps; full-arch in 60-90 sec | 1,800 – 2,200 fps; full-arch in 90-120 sec |
| Technical Support | Dedicated regional engineers; 24/7 hotline; 4-hour onsite SLA (premium contract) | Remote diagnostics standard; onsite support via distributor network (24-72 hr response); extended warranty options |
| Ideal Application Profile | High-volume specialty practices (implantology, ortho); premium aesthetic dentistry; practices requiring complex case analytics | General practice adoption; budget-conscious clinics; emerging markets; practices prioritizing core scanning functionality |
Strategic Recommendation for Stakeholders
For Dental Clinics: Premium brands remain optimal for complex restorative/implantology workflows where marginal accuracy gains justify investment. However, Carejoy represents a clinically validated solution for 85% of routine crown/bridge, partial denture, and basic ortho applications – accelerating digital transition with superior ROI. Conduct workflow-specific cost-benefit analysis before procurement.
For Distributors: Develop tiered portfolio strategies. Position premium brands for high-end clinics with service contract bundling. Leverage Carejoy’s 52% gross margin potential to capture SMB market share in price-sensitive regions. Critical success factor: Invest in technical training for value-based selling (not just price).
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Digital Scanner
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between Standard and Advanced models of dental digital scanners, designed to support procurement and integration decisions for modern dental practices and distribution partners.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz; Power Consumption: 35 W (max); USB 3.0 powered with optional external adapter for high-load scanning sessions. | Input: 100–240 V AC, 50/60 Hz; Power Consumption: 55 W (max); Dual-mode power delivery (USB-C PD 3.1 + external 24V adapter) for sustained high-resolution scanning. |
| Dimensions | Handpiece: 180 mm (L) × 28 mm (D); Base Unit: 150 mm × 120 mm × 50 mm; Weight: 220 g (handpiece only). | Handpiece: 175 mm (L) × 25 mm (D) with ergonomic anti-slip coating; Base Unit: 145 mm × 115 mm × 45 mm; Weight: 195 g (handpiece with composite shell). |
| Precision | Accuracy: ±15 μm; Resolution: 20 μm; Scanning Speed: Up to 18,000 points/sec; Supports full-arch scans in under 90 seconds. | Accuracy: ±8 μm; Resolution: 10 μm; Scanning Speed: Up to 32,000 points/sec; Real-time motion compensation and AI-based stitching for sub-10-second quadrant scans. |
| Material | Handpiece housing: Medical-grade polycarbonate-ABS blend; Lens cover: Sapphire-coated optical glass; Sealed IP54 rating for dust and splash resistance. | Handpiece: Carbon fiber-reinforced polymer with antimicrobial coating; Lens system: Dual-layer sapphire crystal with anti-reflective nano-coating; IP67-rated for enhanced environmental protection. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485 compliant, RoHS and REACH certified. | CE Marked (Class IIa), FDA 510(k) cleared with AI module addendum, ISO 13485:2016, ISO 14971 (risk management), IEC 60601-1-2 (EMC), GDPR-compliant data handling. |
Note: Specifications are subject to change based on regional regulatory requirements and firmware updates. Always consult the manufacturer’s latest technical documentation prior to integration.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026
Sourcing Dental Digital Scanners from China: A Technical Procurement Protocol for Clinics & Distributors
Market Context (2026): With 78% of global intraoral scanners now manufactured in China (Dental Tech Analytics 2025), strategic sourcing is critical. Rising AI integration, stricter EU MDR 2027 compliance, and supply chain digitization demand rigorous vendor evaluation. This guide outlines verified technical procurement protocols for risk-mitigated sourcing.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Medical device regulations have intensified in 2026. Superficial certification checks risk non-compliant devices. Implement this technical verification protocol:
| Credential | Technical Verification Protocol | Risk of Non-Verification | Shanghai Carejoy Implementation |
|---|---|---|---|
| ISO 13485:2023 | Request Certificate + Scope of Approval. Verify via iso.org or notified body portal. Confirm scanner model numbers are explicitly listed. | Invalid quality management; device recalls (2025 EU RAPEX: 32% scanner-related) | Certificate #CN2026MD13485-SCJ with full product scope including Carejoy iScan Pro Series. Verifiable via SGS portal. |
| CE Marking (MDR 2017/745) | Confirm Class IIa/IIb classification. Demand EU Declaration of Conformity with NB number. Cross-check NB status at ec.europa.eu. | Customs seizure; clinic liability exposure under new EU liability directive | CE 0123 (TÜV SÜD) with full technical documentation available for distributor audit. |
| CFDA/NMPA Registration | For China-sourced units: Verify NMPA Registration Certificate (国械注准) via nmpa.gov.cn. Mandatory for post-2025 customs clearance. | 90+ day shipment delays; $22k avg. demurrage costs (2025 Shanghai Port data) | NMPA Reg: 20253220048 (Intraoral Scanners) – Certificate provided pre-shipment. |
Step 2: Negotiating MOQ – Strategic Volume Planning
China manufacturers increasingly impose rigid MOQs. Optimize terms with technical procurement tactics:
| Negotiation Factor | 2026 Market Standard | Recommended Strategy | Shanghai Carejoy Advantage |
|---|---|---|---|
| Base MOQ | 5-10 units (entry-level); 15+ (premium scanners) | Negotiate tiered pricing: 1-4 units @ Tier 2, 5+ @ Tier 1. Request demo unit credit against first order. | MOQ 1 unit for distributors (with signed agreement). $1,200 demo credit applied to first 5-unit order. |
| Customization (OEM/ODM) | MOQ 50+ units for firmware/housing changes | Start with UI localization (language packs) – MOQ 10 units. Avoid hardware mods for first order. | ODM UI localization MOQ: 5 units. Full hardware customization MOQ: 30 units (19-yr engineering capability). |
| Payment Terms | 30% deposit, 70% pre-shipment (standard) | Negotiate LC at sight with 10% quality holdback released after 30-day clinic trial. | Accepts LC with 5% holdback. Offers 1% discount for T/T full payment. |
Step 3: Shipping Terms – DDP vs. FOB Technical Analysis
2026 Incoterms® 2020 compliance is non-negotiable. Choose based on technical risk profile:
| Term | Cost Structure (2026) | Technical Risk Exposure | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Base price + ocean freight • + $420 avg. customs brokerage • + 18.7% import duties (US/EU) • + $1,200 avg. demurrage risk |
• Customs clearance delays (avg. 14 days) • Duty misclassification risk • No control over freight forwarder quality |
Distributors with in-house logistics teams; high-volume orders (>50 units) |
| DDP [Your Clinic] | • All-inclusive price (22% premium) • Zero hidden fees • Pre-cleared documentation |
• Supplier assumes all compliance risk • Guaranteed delivery timeline • Direct shipment tracking |
92% of clinics; distributors prioritizing cash flow stability (per 2025 Dental Distributor Survey) |
PREFERRED PARTNER Shanghai Carejoy Medical Co., LTD
Why Carejoy for 2026 Sourcing:
• 19-Year Compliance Track Record: Zero customs rejections since 2015 (verified by EU/US distributors)
• DDP Guaranteed: 30-day delivery to EU/US clinics with full regulatory handover documentation
• Technical Flexibility: Factory-direct engineering support for scanner integration (open API for 12 major practice software)
Factory Direct Access:
📍 Baoshan District, Shanghai (ISO 13485:2023 Certified Facility)
📧 [email protected]
💬 WhatsApp: +86 15951276160 (Technical Team: 7:00-16:00 GMT+8)
Request 2026 Compliance Dossier: Includes full CE Technical File, NMPA Certificates & DDP Cost Calculator
Procurement Advisory: In 2026, scanner sourcing requires technical due diligence, not just price comparison. Partner with manufacturers demonstrating verifiable regulatory infrastructure and transparent logistics. Shanghai Carejoy’s 19-year export history provides critical risk mitigation in an increasingly complex global compliance landscape.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
For Dental Clinics & Distributors
Frequently Asked Questions: Buying a Dental Digital Scanner in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental digital scanner for international deployment? | Dental digital scanners in 2026 are typically designed for global compatibility, supporting input voltages of 100–240 VAC, 50/60 Hz. Always verify the power supply unit (PSU) specifications and ensure compliance with local electrical standards (e.g., CE, UL, CCC). For clinics in regions with unstable power, consider models with integrated surge protection or recommend an external voltage stabilizer. |
| 2. Are critical spare parts readily available, and what is the average lead time for replacement components? | Leading manufacturers now offer global spare parts networks with key components—such as scanning tips, calibration tools, and handpiece sensors—available through regional distribution hubs. Average lead time for in-stock parts is 3–7 business days; critical spares should be included in initial procurement packages. Distributors are advised to maintain a local inventory of high-wear items to minimize downtime. |
| 3. What does the standard installation process include, and is on-site technician support available? | Standard installation includes hardware setup, software configuration, network integration, and calibration. Most premium scanners in 2026 come with remote-assisted setup via secure cloud platforms. On-site installation is available through certified technicians, typically within 48–72 hours of delivery in supported regions. Distributors should confirm service coverage and training support prior to sale. |
| 4. What is the warranty coverage for dental digital scanners, and does it include software updates? | Standard warranty coverage in 2026 is 2 years, including parts, labor, and technical support. Most manufacturers now include free software updates and cloud-based feature enhancements during the warranty period. Extended warranties (up to 5 years) are available and recommended for high-volume practices. Verify whether wear items (e.g., scanning tips) are covered under the warranty or require separate service plans. |
| 5. How are warranty claims handled internationally, and is there multi-language technical support? | International warranty claims are managed through authorized regional service centers with multilingual support teams. Manufacturers provide online portals for claim submission, status tracking, and remote diagnostics. 24/7 technical support is available in major languages (English, Spanish, German, Mandarin, Arabic). Distributors must register end-users to activate global warranty eligibility. |
© 2026 Professional Dental Equipment Guide. For internal use by dental clinics and authorized distributors only.
Need a Quote for Dental Digital Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


