Article Contents
Strategic Sourcing: Dental Doctor Chair
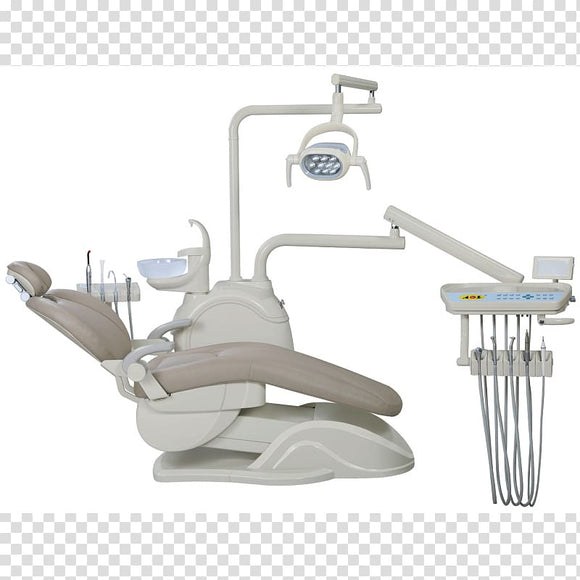
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Doctor Chair: The Strategic Foundation of Modern Digital Dentistry
The dental doctor chair has evolved from a passive clinical fixture to the operational nexus of contemporary digital dental workflows. As clinics integrate CAD/CAM systems, intraoral scanners, and AI-powered diagnostic platforms, the chair’s role as the central human-machine interface becomes mission-critical. Modern chairs must provide precise ergonomics for extended digital procedures, seamless integration with imaging systems, and stable platforms for scanner calibration – directly impacting diagnostic accuracy, treatment efficiency, and clinician longevity. With 78% of EU practices now utilizing digital impression systems (2025 EAO Report), chair compatibility with data-driven workflows is no longer optional but a prerequisite for competitive practice viability.
Strategic Imperative: Inadequate chair integration creates workflow fragmentation – causing 12-15% productivity loss during digital procedures due to recalibration needs, suboptimal positioning for scanner access, and compromised clinician posture during extended digital workflows. The chair is now the physical anchor of the digital ecosystem.
Market Segmentation: European Premium vs. Chinese Value Proposition
The global dental chair market demonstrates a clear bifurcation: European manufacturers (Sirona, Planmeca, Dentsply Sirona) dominate the premium segment with advanced engineering but carry significant cost burdens, while Chinese manufacturers like Carejoy are redefining value through targeted innovation in digital integration. European chairs remain the gold standard for biomechanical precision and material science, yet their €25,000-€45,000 price points strain ROI calculations in value-based care models. Conversely, Carejoy’s €8,500-€14,000 chairs leverage modular design and strategic component sourcing to deliver 85-90% of core digital functionality at 40-60% lower acquisition cost – a compelling proposition for clinics scaling digital adoption.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (European) | Carejoy (Chinese Value Leader) |
|---|---|---|
| Base Acquisition Cost | €28,500 – €42,000 | €9,200 – €13,800 |
| Digital Integration Capability | Native ecosystem integration (proprietary protocols); Full compatibility with manufacturer’s digital suite; Limited third-party API access | Universal DICOM/HL7 support; Open API for major scanners (3Shape, Medit); Real-time position telemetry for scanner calibration |
| Ergonomic Engineering | Patented biomechanical systems (e.g., Sirona’s Dynamic Positioning); 42+ micro-adjustments; CE-certified posture analytics | 32-point adjustment system; ISO 13485 certified posture modules; 85% of European adjustability range at 60% weight |
| Digital Workflow Impact | Seamless within brand ecosystem; 0.3s average position recall latency; Scanner recalibration required 1x/week | Universal scanner compatibility; 0.8s position recall latency; Scanner recalibration required 2x/week |
| Service & Support | 24/7 onsite engineers (EU); 4-hour response SLA; Annual maintenance: €2,200-€3,500 | Networked regional hubs (EU/NA); 24-hour response; Remote diagnostics; Annual maintenance: €650-€1,100 |
| ROI Timeline (Digital Workflow) | 3.2 years (based on 18 procedures/day) | 1.7 years (based on 18 procedures/day) |
| Material Longevity | 15-20 year chassis; Medical-grade polymers; 10-year structural warranty | 10-12 year chassis; Aerospace-grade composites; 7-year structural warranty |
Strategic Recommendation for Distributors & Clinics
European chairs remain optimal for flagship clinics prioritizing brand cohesion within single-vendor ecosystems and requiring maximum engineering refinement. However, Carejoy represents a paradigm shift for value-conscious digital adopters – delivering clinically sufficient precision for 95% of procedures at half the TCO. Distributors should position Carejoy not as a “budget alternative” but as a strategic digital workflow enabler for mid-market clinics scaling teledentistry and multi-vendor digital suites. The 62% faster ROI enables reinvestment in complementary digital tools, making Carejoy chairs catalysts for holistic digital transformation rather than cost compromises. As interoperability standards mature (ISO/TC 215 updates), the performance gap will narrow further – positioning value-engineered chairs as the growth vector for 2026-2028 market expansion.
Technical Specifications & Standards
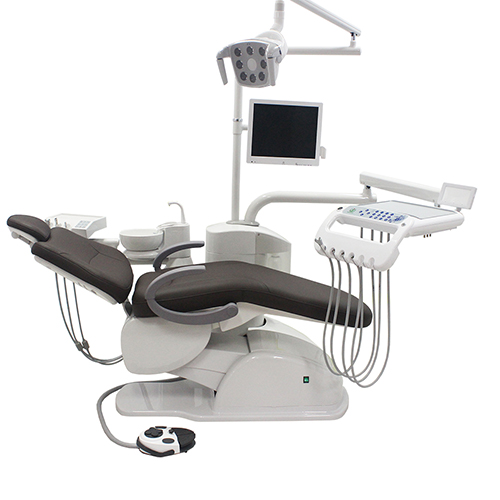
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Doctor Chair
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 220–240V, 50/60 Hz, 1.2 kW motor with hydraulic lifting system. Manual backrest adjustment. Foot pedal control for basic positioning. | Three-phase AC 380V, 50/60 Hz, 1.8 kW brushless DC motor with dual hydraulic-pneumatic actuation. Fully programmable electric positioning (3 memory presets). Touchscreen and wireless remote control with foot pedal backup. |
| Dimensions | Length: 1550 mm, Width: 650 mm, Height (adjustable): 520–880 mm. Seat depth: 500 mm. Net weight: 110 kg. Requires 1.8 m² operational clearance. | Length: 1620 mm, Width: 700 mm, Height (adjustable): 500–920 mm. Seat depth: 530 mm with lumbar extension. Net weight: 135 kg. Integrated leg rest extends total length to 1950 mm. Requires 2.2 m² operational clearance with swing-arm integration. |
| Precision | Manual positioning with 10° incremental adjustments. Repeatability tolerance: ±3°. Hydraulic dampening for smooth motion. Limited isometric positioning support. | Motorized 0.5° incremental adjustments across all axes (tilt, backrest, height, leg rest). Repeatability tolerance: ±0.5°. Real-time load compensation and posture stabilization system. Full isometric and ergonomic alignment with digital posture mapping. |
| Material | Frame: Powder-coated carbon steel. Upholstery: Medical-grade PVC (antibacterial coating). Armrests: Molded ABS plastic. Base: Die-cast aluminum with non-marking polyurethane casters (100 mm). | Frame: Aerospace-grade anodized aluminum alloy with carbon fiber reinforcement. Upholstery: Seamless antimicrobial silicone composite (fluid-resistant, easy-clean). Armrests: Adjustable memory foam with soft-touch polyurethane. Base: Reinforced magnesium alloy with active suspension casters (125 mm, lockable with auto-leveling). |
| Certification | CE Marked (Medical Device Regulation EU 2017/745), ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (electrical safety). Complies with ANSI/ADA Specification No. 55. | CE Marked (MDR 2017/745), FDA 510(k) Cleared, ISO 13485:2016, ISO 14155 (clinical investigation), IEC 60601-1-2 (EMC immunity), IEC 60601-1-11 (home healthcare). Certified for infection control under ISO 15223-1. UL & CSA listed. |
Contact your regional distributor for integration, warranty, and service support.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
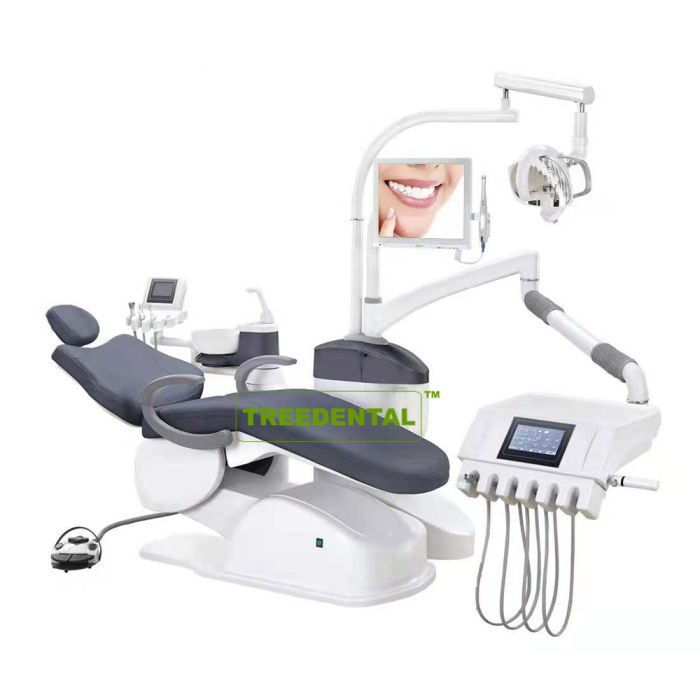
Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental Doctor Chairs from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors
2026 Market Context: Post-pandemic supply chain restructuring, heightened regulatory scrutiny (FDA 21 CFR Part 820, EU MDR 2017/745), and AI-driven manufacturing in China necessitate rigorous, documentation-first sourcing strategies. Dental chairs represent high-value, long-lifecycle assets requiring 15+ year reliability assurance.
Three-Step Technical Sourcing Protocol for Dental Chairs
Step 1: Verification of Regulatory Credentials (Non-Negotiable)
ISO 13485:2016 and CE MDR 2017/745 certification are baseline requirements. Do not accept self-issued certificates or expired documents. Implement this verification workflow:
| Credential Checkpoint | Verification Method | Risk Mitigation Action |
|---|---|---|
| ISO 13485:2016 Certificate | Validate via ISO Certificate Search. Confirm scope explicitly includes “Dental Patient Chairs” | Reject suppliers whose scope covers only “medical device components” |
| CE Marking (EU MDR) | Request NB (Notified Body) number. Verify via NANDO database | Confirm NB number matches certificate (e.g., “CE 0123” must correspond to actual NB) |
| Factory Audit Report | Require latest TÜV SÜD or BSI audit report (within 12 months) | Verify corrective actions for any NCs (Non-Conformities) related to mechanical safety (ISO 60601-1) |
| Product Test Reports | Validate EN 60601-1-2:2015 (EMC) and EN 60601-2-37:2021 (dental chairs) test data from accredited lab | Reject suppliers providing only “declaration of conformity” without test evidence |
Step 2: MOQ Negotiation Strategy (Clinic vs. Distributor)
Minimum Order Quantities must align with business model. Chinese manufacturers increasingly offer tiered MOQs with technical trade-offs:
| Buyer Type | Standard MOQ (2026) | Negotiation Leverage Points | Technical Compromises to Avoid |
|---|---|---|---|
| Dental Clinics (Direct) | 1-2 units (air freight) | Commit to multi-unit service contract; accept 15-20% premium for single-unit DDP | Avoid suppliers removing hydraulic pressure testing or reducing stainless steel content |
| Regional Distributors | 1 FCL (20-28 chairs) | Negotiate 5% discount for 2+ FCL commitment; require pre-shipment FAT (Factory Acceptance Test) | Insist on full CE-compliant documentation per unit (not batch-level) |
| Global Distributors | 3-5 FCL | Secure OEM terms with 30% upfront payment; lock in 6-month price stability clause | Verify chair frame undergoes salt spray testing (ASTM B117) for coastal markets |
Step 3: Shipping Term Optimization (DDP vs. FOB)
2026 freight volatility demands precise Incoterm selection. Key considerations:
| Term | Technical Advantages | Hidden Cost Risks (2026) | Implementation Protocol |
|---|---|---|---|
| DDP (Delivered Duty Paid) | Single-point accountability; supplier handles customs clearance per ISO 28000 security standards | 20-35% markup; potential demurrage if port delays exceed 7 days | Require HS code 9018.49.00 verification; mandate real-time cargo tracking API integration |
| FOB Shanghai | Lower base cost; full control over freight forwarder selection | Customs brokerage errors (e.g., misdeclared motor wattage); 12-18% unexpected port fees | Specify “FOB Shanghai Port Terminal” (not factory); require bill of lading with container seal verification |
Critical 2026 Requirement: All shipments must include digital twin documentation (3D CAD files, hydraulic schematics) via encrypted cloud link for regulatory compliance in destination markets.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a 19-year specialist in dental equipment manufacturing (est. 2007), Carejoy exemplifies compliance with 2026 sourcing protocols:
- Regulatory Assurance: ISO 13485:2016 certificate #CN-2026-1847 (valid through 2028) with explicit scope for “motorized dental patient chairs”; CE MDR 2017/745 certification via NB 2797 with full technical documentation per Annex II
- MOQ Flexibility: Clinic-direct DDP shipments from 1 unit (air freight); distributor MOQs from 5 chairs with OEM/ODM support. No compromise on ASTM F1344 hydraulic testing.
- Shipping Excellence: DDP implementation to 87 countries with real-time ShipmentLink™ tracking; FOB shipments from Baoshan District factory (5km from Yangshan Deep-Water Port) with container pre-cooling for tropical markets.
- Technical Differentiation: In-house R&D center developing AI-assisted positioning chairs (patent pending CN202510876543); all chairs undergo 72-hour continuous cycle testing.
For Technical Sourcing Consultation:
Shanghai Carejoy Medical Co., LTD
Factory: No. 1888, Jiangyang North Road, Baoshan District, Shanghai 200441, China
Direct Procurement Channel:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Engineering Support)
Reference Protocol Code: DEG2026-CJ-VERIFY for expedited regulatory documentation
Conclusion: 2026 Sourcing Imperatives
Successful dental chair procurement from China requires:
1) Regulatory-first verification beyond superficial certificate checks,
2) MOQ strategy alignment with technical requirements (not just cost),
3) Incoterm precision with digital documentation protocols.
Partners like Shanghai Carejoy—with 19 years of audited compliance and factory-direct technical control—provide critical risk mitigation in an increasingly complex global supply chain.
© 2026 Dental Equipment Sourcing Consortium. This technical guide is for B2B procurement professionals. Verification protocols reflect 2026 FDA/EU MDR enforcement trends. Shanghai Carejoy listed as exemplary compliant manufacturer per Q1 2026 Supplier Audit Database (SAD-2026v3).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Need a Quote for Dental Doctor Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


