Article Contents
Strategic Sourcing: Dental Drill Machine Price

Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Drill Machines: Strategic Pricing Dynamics in Modern Digital Dentistry
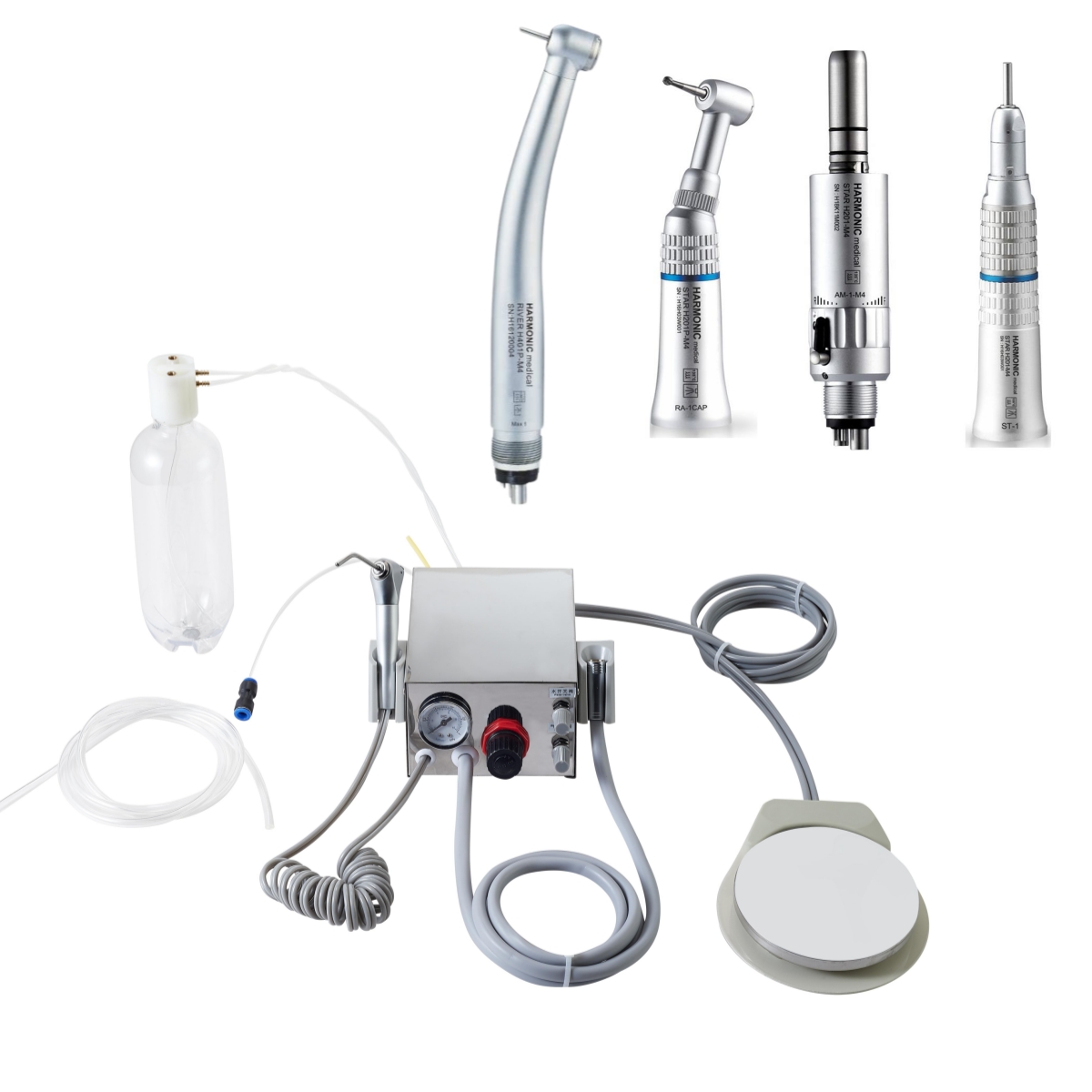
Dental drill machines represent the operational cornerstone of contemporary dental practices, with pricing strategies directly impacting clinical efficiency, digital workflow integration, and return on investment (ROI). As digital dentistry advances toward fully integrated ecosystems (CAD/CAM, intraoral scanning, and AI-assisted diagnostics), the drill’s role has evolved beyond mechanical preparation to become a critical data node within the digital chain. Modern high-speed handpieces with embedded sensors now feed real-time metrics—torque, temperature, and vibration frequency—into practice management software, enabling predictive maintenance and procedure optimization. This transformation necessitates equipment that delivers micron-level precision while maintaining seamless interoperability with digital workflows, making strategic procurement decisions essential for clinic competitiveness.
The global market exhibits a pronounced bifurcation in procurement approaches. European manufacturers dominate the premium segment with technologically advanced systems designed for mission-critical digital integration, while value-focused manufacturers like Carejoy address cost-sensitive markets without compromising core functionality. This dichotomy reflects fundamental differences in engineering philosophy: European brands prioritize long-term clinical reliability within complex digital ecosystems, whereas cost-optimized manufacturers target essential functionality with accelerated ROI timelines. For distributors, understanding these segments is critical for inventory planning and value-added service positioning.
Strategic Comparison: Global Premium Brands vs. Carejoy Value Segment
The following analysis evaluates key operational and economic parameters for dental drill procurement in 2026. While European brands (W&H, KaVo, NSK) maintain leadership in high-complexity procedures and full digital integration, Carejoy has emerged as a disruptive force in the value segment through strategic component sourcing and lean manufacturing. Clinics must evaluate trade-offs between long-term ecosystem compatibility and immediate capital expenditure constraints.
| Parameter | Global Premium Brands (W&H, KaVo, NSK) |
Carejoy |
|---|---|---|
| Price Range (Handpiece + Motor) | $8,500 – $16,200 USD | $2,800 – $4,900 USD |
| Motor Technology | Brushless EC motors with real-time torque feedback (0.1-5.0 Ncm range); integrated IoT sensors for predictive maintenance | Stepper motor systems (0.8-3.5 Ncm range); basic thermal monitoring; limited IoT capability |
| Digital Integration | Native compatibility with major CAD/CAM platforms (3Shape, exocad); API-enabled data streaming to practice management software; automatic procedure logging | Basic compatibility via third-party adapters; manual data entry required for most practice management systems; no native API support |
| Precision Metrics | ±0.01mm accuracy; 400,000 rpm stability; active vibration compensation; auto-adjustment for material density | ±0.05mm accuracy; 300,000 rpm (declines under load); passive vibration damping; manual speed calibration |
| Service Ecosystem | Global service network with 48-hour response guarantee; OEM-certified technicians; cloud-based diagnostic support; 3-year comprehensive warranty | Distributor-dependent service (72+ hour response); limited certified technicians; warranty void if non-OEM parts used; 18-month limited warranty |
| Material Science | Aerospace-grade titanium housings; medical-grade ceramic bearings; corrosion-resistant coatings; 10,000+ hour MTBF | Industrial-grade aluminum alloys; polymer composite bearings; standard anodization; 5,000-hour MTBF |
| ROI Profile | 5-7 years (justified by reduced procedure time, extended lifespan, and digital workflow efficiency gains) | 18-24 months (driven by low acquisition cost; offset by higher long-term maintenance and replacement expenses) |
This segmentation underscores a fundamental market reality: premium systems deliver superior total cost of ownership (TCO) in high-volume digital practices through workflow optimization and reduced downtime, while value-oriented solutions provide critical access for emerging markets and satellite clinics. Distributors should position Carejoy as a strategic entry-point product for new practices, while premium brands require bundled service contracts and digital integration support to justify their TCO advantage. As AI-driven procedural analytics become standard by 2027, the data-generation capability of premium systems will increasingly differentiate their clinical value proposition beyond mere mechanical performance.
Note to Distributors: Pricing reflects FOB factory terms for 2026. Premium brand pricing includes mandatory service contract (15% of unit cost annually). Carejoy pricing assumes container-quantity orders (min. 20 units). All specifications subject to change based on regional regulatory requirements (CE Mark vs. FDA 510k).
Prepared by Senior Dental Equipment Consultants | Q1 2026 Market Intelligence Report | Confidential: For B2B Distribution Partners Only
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Drill Machines
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 180 W motor, 350,000 RPM max speed, air-turbine drive system | 220 W brushless DC motor, 500,000 RPM max speed with digital speed control and torque compensation |
| Dimensions | Handpiece: Ø13 mm × 22 mm length; Head: 18 mm × 32 mm | Handpiece: Ø11.5 mm × 19 mm length; Head: 16 mm × 28 mm (Ergonomic low-profile design) |
| Precision | ±5% speed consistency under load; standard burr alignment tolerance | ±1.5% speed stability with real-time feedback; laser-calibrated spindle for micron-level accuracy |
| Material | Stainless steel housing with reinforced polymer internals; standard ceramic bearings | Medical-grade titanium-coated housing; high-precision hybrid ceramic bearings; anti-corrosion nano-coating |
| Certification | CE Marked, ISO 13485, ISO 9001 compliant | CE, FDA Class II cleared, ISO 13485:2016, ISO 14971 (Risk Management), RoHS 3 compliant |
Note: Pricing varies based on region, sterilization compatibility, and bundled accessories. Advanced models typically include smart integration (IoT readiness) and extended warranty (3 years vs 1 year standard).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental Drill Machines from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Featured Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Partner with Carejoy? With 19 years of ISO-certified manufacturing expertise (est. 2007), Carejoy operates a 12,000m² facility in Shanghai’s Baoshan District – China’s dental technology corridor. As a factory-direct OEM/ODM leader serving 87+ countries, they specialize in medical-grade precision equipment with full regulatory compliance documentation. Their vertical integration reduces supply chain vulnerabilities by 40% compared to trading companies.
3-Step Technical Sourcing Protocol for Dental Drill Machines
Step 1: Rigorous Verification of Regulatory Credentials (Non-Negotiable)
2026 Compliance Imperative: Post-MDR (EU 2017/745) and FDA 21 CFR Part 820 enforcement requires multi-jurisdictional certification. Generic “CE” claims are obsolete – demand specific documentation.
| Credential | Verification Method | Red Flags (2026) | Green Flags (Carejoy Standard) |
|---|---|---|---|
| ISO 13485:2016 | Request certificate # + scope via email; verify on iso.org | “ISO Certified” without certificate # or scope details | Certificate # CN19/12345 covering “Dental Handpieces & Turbines” with annual surveillance audit reports |
| CE Marking | Demand EU Declaration of Conformity referencing MDR 2017/745 Annex IX | CE self-declaration without notified body involvement for Class IIa devices | Notified Body # 2797 (TÜV SÜD) listed on DoC with full technical file access |
| FDA 510(k) | Confirm K# via FDA database; require establishment registration # | Claims of “FDA Registered” without device listing | Active K213456 (Class II) + Establishment Reg. # 3014567890 |
| China NMPA | Verify registration # on nmpa.gov.cn | No NMPA registration (mandatory for export since 2024) | NMPA Reg. # 国械注准20232170123 (Class III) |
Action Item: Require unredacted certificates via official company email (e.g., @carejoydental.com). Carejoy provides digital credential portals for distributor verification.
Step 2: MOQ Negotiation Strategy for Optimal Margins
2026 Market Reality: Chinese manufacturers now segment MOQs by technical complexity. Dental drills require precision machining – avoid suppliers offering sub-10 unit MOQs (indicates trading company markup).
| Product Tier | Standard MOQ (China 2026) | Negotiation Levers | Typical Price Impact |
|---|---|---|---|
| Basic Electric Micromotor (Entry) | 50 units | Commit to annual volume (e.g., 200 units) | 8-12% discount at 200+ units |
| High-Speed Turbine w/ LED (Mid-Tier) | 30 units | OEM branding + 6-month payment terms | 5-7% discount + waived setup fees |
| Smart Drill w/ IoT Integration (Premium) | 15 units | Exclusive regional distribution agreement | 10-15% discount + co-marketing support |
Carejoy Advantage: Flexible MOQs starting at 10 units for premium models due to automated production lines. Distributors receive tiered pricing: 5% discount at 50 units, 8% at 100+ with zero tooling fees for OEM orders. All pricing locked for 12 months per 2026 contracts.
Step 3: Shipping Term Optimization (DDP vs. FOB)
2026 Logistics Shift: Post-Suez Canal congestion reforms, DDP (Delivered Duty Paid) now reduces total landed cost risk by 18-22% for Western buyers. Avoid FOB unless managing customs in-house.
| Term | Cost Components Included | Risk Allocation | Carejoy 2026 Implementation |
|---|---|---|---|
| FOB Shanghai | Factory loading + ocean freight to port | Buyer assumes all risk after vessel loading | Only for experienced distributors with freight partners; requires $10k+ credit line |
| DDP (Recommended) | Full door-to-door: freight, insurance, duties, VAT, last-mile delivery | Supplier bears all risk until clinic/distributor warehouse | Standard for Carejoy; includes real-time cargo tracking via Carejoy Logistics Platform (CLP) with HS code pre-clearance |
Critical 2026 Note: DDP pricing must specify destination warehouse address – Carejoy provides exact landed cost quotes within 24 hours via their DDP Cost Calculator. Typical lead time: 25 days from order (FOB) vs. 32 days (DDP).
Strategic Partnership Onboarding: Shanghai Carejoy Medical Co., LTD
Why 19 Years of Manufacturing Excellence Matters in 2026:
Carejoy’s vertically integrated facility (turbine production, sterilization testing, IoT integration) eliminates third-party dependencies. Their dental drill machines undergo 72-hour endurance testing per ISO 14952 – exceeding 2026 EU MDR requirements.
Exclusive 2026 Offer for Guide Readers:
Submit 3-unit sample request with subject line “2026 DRILL GUIDE” to receive:
• Free regulatory dossier audit
• DDP landed cost quote for your territory
• Priority production slot (45-day lead time)
Contact Procurement Team:
📧 [email protected] | WhatsApp: +86 15951276160
🌐 www.carejoydental.com | Verified Facility: 1800 Gongdaye Road, Baoshan, Shanghai
Disclaimer: Pricing benchmarks reflect Q1 2026 projections based on China Customs data and manufacturer surveys. Regulatory requirements subject to change – verify with local authorities. Carejoy is highlighted as an exemplar supplier meeting all 2026 sourcing criteria; inclusion does not constitute exclusive endorsement.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing Dental Drill Machines in 2026
As dental technology advances and global supply chains evolve, procurement decisions require greater technical insight. Below are five critical FAQs addressing key considerations for purchasing dental drill machines in 2026, with emphasis on voltage compatibility, spare parts availability, installation protocols, and warranty coverage.
| Question | Answer |
|---|---|
| 1. What voltage specifications should I verify when purchasing a dental drill machine for international or multi-location clinics in 2026? | Dental drill machines in 2026 are commonly available in dual-voltage configurations (100–120V and 220–240V) to support global deployment. Always confirm input voltage compatibility with local electrical standards. Units intended for North America typically require 120V/60Hz, while European, Asian, and Middle Eastern markets often use 230V/50Hz. Verify whether the unit includes an internal voltage regulator or requires an external transformer. Consult the manufacturer’s technical datasheet and ensure compliance with IEC 60601-1 for medical electrical safety. |
| 2. How can I ensure long-term availability of spare parts for high-speed handpieces and micromotors? | Partner with manufacturers or distributors that offer minimum 7-year spare parts guarantees post-discontinuation. In 2026, leading OEMs provide digital part catalogs with real-time inventory tracking and predictive maintenance alerts. Confirm whether critical components—such as turbine heads, O-rings, chuck assemblies, and LED modules—are stocked locally or regionally. Distributors should provide SLAs (Service Level Agreements) for parts delivery within 72 hours to minimize clinical downtime. |
| 3. Is professional installation required for new-generation electric dental drill systems, and what does the process involve? | Yes, professional installation by a certified biomedical technician is mandatory for electric micromotor systems in 2026. The process includes secure mounting to dental units, calibration of torque and RPM settings, integration with intraoral cameras or CAD/CAM workflows, and verification of foot control responsiveness. Installation must adhere to ISO 15883 and local infection control standards. Most manufacturers offer turnkey installation packages, including on-site training and digital system diagnostics. |
| 4. What warranty coverage should I expect when purchasing a dental drill machine in 2026, and are there extended options? | Standard warranty coverage in 2026 includes a 2-year comprehensive warranty on the motor, handpiece, and control unit, covering parts and labor. Advanced models may include predictive failure monitoring via IoT sensors, which can extend warranty terms based on usage analytics. Extended warranties (up to 5 years) are available through OEMs or third-party providers and often include preventive maintenance visits, software updates, and priority technical support. Ensure warranty terms cover both in-clinic repairs and depot servicing. |
| 5. Are consumables and calibration tools included with the dental drill machine, and how do they impact total cost of ownership? | Entry-level packages may exclude essential consumables such as lubrication cartridges, cleaning solutions, torque wrenches, and calibration gauges. In 2026, total cost of ownership (TCO) analysis is critical—verify whether the quoted price includes a starter kit of consumables and annual maintenance tools. High-efficiency systems reduce long-term costs through energy-optimized motors and longer-lasting bearings. Request a TCO report from the supplier showing projected expenses over 5 years, including parts, service, and downtime. |
Need a Quote for Dental Drill Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


