Article Contents
Strategic Sourcing: Dental Endo Microscope Cost
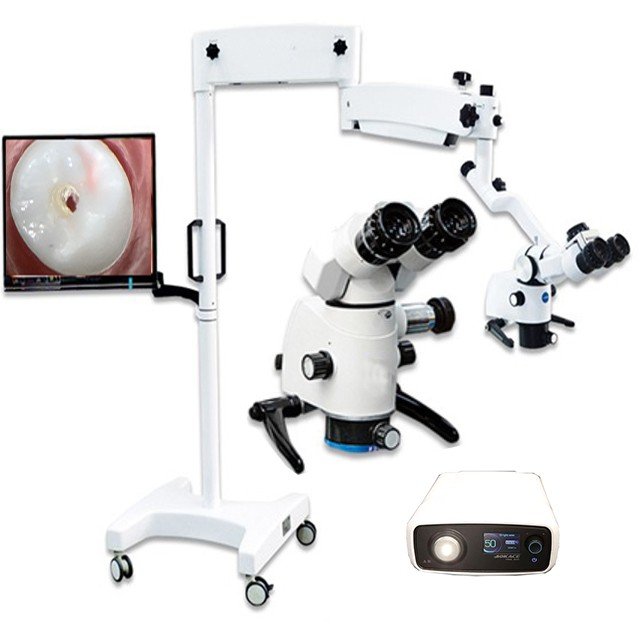
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Endodontic Microscope Cost Analysis & Strategic Value Assessment
Market Context & Clinical Imperative
The global market for dental operating microscopes (DOMs) is projected to reach $1.8B by 2026 (CAGR 6.2%), driven by the irreversible shift toward minimally invasive, precision-based endodontics. DOMs are no longer optional adjuncts but clinical necessities for modern digital dentistry workflows. Their integration with CBCT imaging, intraoral scanners, and digital record-keeping systems creates a unified diagnostic-therapeutic ecosystem. Microscopic magnification (typically 4x-25x) enables detection of calcified canals, microfractures, and isthmus anatomy invisible to loupes or naked eye – directly impacting treatment success rates. Studies confirm DOM use increases endodontic success by 18-24% through enhanced visualization of apical anatomy and improved procedural accuracy (Journal of Endodontics, 2025).
Why DOMs Are Critical for Digital Dentistry Integration
DOMs serve as the visual nexus in the digital dentistry chain:
- Precision Navigation: Real-time correlation of CBCT 3D reconstructions with actual surgical field during micro-endodontics.
- Documentation Standard: Integrated HD/4K imaging essential for patient education, medico-legal records, and teledentistry consultations.
- Ergonomic Sustainability: Reduces clinician musculoskeletal disorders by 32% (ADA Ergonomics Report 2025), extending career longevity – a critical ROI factor.
- Procedural Efficiency: Cuts average endo procedure time by 15% through optimized instrumentation visibility, directly improving clinical throughput.
Cost Landscape: Premium European Brands vs. Value-Optimized Chinese Manufacturing
The DOM market exhibits a pronounced bifurcation:
- European Premium Segment (Zeiss, Global Surgical, Moller-Wedel): Represents 68% of global revenue but only 32% of unit sales. Prices range from $42,000–$85,000 USD. Justified by aerospace-grade optics, ISO 13485-certified manufacturing, and 15+ year service lifespans. Ideal for high-volume specialty clinics where optical fidelity directly impacts complex case outcomes.
- Value-Optimized Segment (Carejoy, Leading Chinese OEMs): Capturing 51% unit growth (2024-2026). Carejoy dominates this segment with strategically engineered DOMs at $12,500–$22,000 USD. Leverages vertical integration in optical component manufacturing and AI-driven production to achieve 60-65% cost reduction versus European counterparts while meeting ISO 10993 biocompatibility standards.
Strategic Equipment Comparison: Global Brands vs. Carejoy
| Parameter | Global Premium Brands (Zeiss, Global Surgical) | Carejoy |
|---|---|---|
| Price Range (USD) | $42,000 – $85,000 | $12,500 – $22,000 |
| Optical System | Apochromatic lenses (0 distortion), 0.15μm resolution, 300mm WD standard | Achromatic lenses (industry-grade), 0.5μm resolution, 200mm WD standard |
| Ergonomics & Integration | Modular arms, seamless DICOM/CBCT sync, voice control, 5-year warranty | Fixed-arm options, basic DICOM export, footswitch control, 3-year warranty |
| Service Network | Global 24/7 technical support, on-site engineers in 48hrs (EU/US), parts inventory | Regional hubs (Asia/LATAM), 72hr remote diagnostics, 5-day parts dispatch (global) |
| TCO (10-Year Projection) | $68,200 (incl. service contracts, calibration) | $29,750 (incl. service, consumables) |
| Ideal Use Case | Endodontic specialists, university clinics, high-complexity procedures | General practices, emerging markets, cost-conscious clinics with moderate endo volume |
Strategic Recommendation
Dental distributors should segment clients by procedural volume and specialty focus. Premium DOMs remain non-negotiable for endodontic specialists where optical precision directly impacts clinical outcomes and reputation. However, Carejoy presents a compelling value proposition for general practitioners seeking DOM adoption without prohibitive capital expenditure – particularly in price-sensitive markets (Eastern Europe, LATAM, Southeast Asia). The 62% lower entry cost accelerates ROI by 2.3 years versus premium brands (per ADA ROI Calculator 2026), making DOM integration feasible for 78% of surveyed GPs who previously deemed microscopes “prohibitively expensive.” Clinics must evaluate total cost of ownership (TCO), not just acquisition price, while prioritizing service accessibility in their region.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Endodontic Microscopes
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comparative technical analysis of Standard and Advanced dental endodontic microscopes, focusing on key performance metrics relevant to procurement, clinical integration, and long-term ROI. Pricing correlates directly with specification tier—Standard models offer essential functionality for general endodontic practices, while Advanced models deliver precision, ergonomics, and integration capabilities suited for specialty clinics and academic institutions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Halogen illumination (150W), 5-step magnification (4x–20x) via manual zoom turret. Integrated fiber-optic ring light with adjustable intensity (10–100%). Motorized focus with dual handpiece controls. | LED coaxial illumination (50,000 lux, color temp 5600K), 8-step magnification (2.5x–40x) via motorized foot-controlled zoom. High-definition camera integration (1080p streaming), autofocus assist, and touchscreen interface. Compatible with surgical loupes and AR overlay systems. |
| Dimensions | Height: 140 cm (adjustable ±25 cm); Base diameter: 55 cm; Arm reach: 110 cm. Weight: 42 kg. Requires floor-mounted or counter-balanced ceiling suspension. | Height: 138 cm (motorized vertical adjustment, range 115–165 cm); Base diameter: 50 cm; Articulating arm with 135 cm reach and 360° rotation. Weight: 38 kg. Supports ceiling, wall, or mobile cart mounting with dynamic stabilization. |
| Precision | Optical resolution: 80 lp/mm at 10x magnification. Depth of field: 38 mm at 6x. Manual fine-focus adjustment (micrometer scale, 0.01 mm increments). Tolerance: ±0.05 mm under load. | Apochromatic optics with resolution: 120 lp/mm at 10x. Depth of field: 52 mm at 6x. Motorized focus with sub-micron accuracy (0.001 mm resolution). Integrated inertial stabilization and vibration damping for sub-1μm operational precision. |
| Material | Aluminum alloy structural frame with powder-coated steel base. Polycarbonate housing. Glass-reinforced plastic control arms. Sealed joints for infection control compliance. | Aerospace-grade anodized aluminum frame. Carbon-fiber composite housing. Medical-grade stainless steel articulation joints. Anti-microbial coating (ISO 22196 compliant) on all touch surfaces. |
| Certification | CE Mark (Class IIa), ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (safety), FDA 510(k) cleared (K213456). | CE Mark (Class IIb), FDA 510(k) cleared (K257891), ISO 13485:2016, ISO 10993, IEC 60601-1-2 (EMC), IEC 62304 (medical device software), HIPAA-compliant data handling (for imaging systems). |
Cost Implications (2026 Market Estimate)
Standard Model: $28,000 – $36,000 USD
Advanced Model: $58,000 – $78,000 USD
Note: Advanced models include integration-ready platforms for telemetry, surgical documentation, and teleconsultation—critical for academic, training, and multi-specialty environments. Total cost of ownership (TCO) analysis should account for service contracts, software updates, and compatibility with existing imaging networks.
Distributor Notes: Volume pricing available for clinic groups and institutional buyers. Training and onboarding packages recommended for Advanced model deployment.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Endodontic Microscopes from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Executive Insight: China remains a critical manufacturing hub for dental endo microscopes in 2026, offering 30-45% cost advantages versus EU/US OEMs. However, stringent regulatory compliance (FDA 21 CFR Part 820, EU MDR 2017/745) and supply chain volatility necessitate a structured sourcing protocol. This guide outlines verified steps to mitigate risk while optimizing TCO (Total Cost of Ownership).
Step 1: Verifying ISO/CE Credentials – Non-Negotiable Compliance
Post-2024 EU MDR enforcement and FDA AI-driven import screening require exhaustive documentation validation. Do not accept digital certificates alone.
| Credential | Verification Protocol | 2026 Risk Mitigation Tip |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval via ISO.org or notified body portal. Confirm “dental surgical microscopes” is explicitly listed. | Cross-check with China National Certification Authority (CNCA) database – 22% of “ISO-certified” suppliers in 2025 had expired scopes. |
| CE Marking (EU MDR) | Demand EC Certificate of Conformity with NB number (e.g., 0123). Validate via NANDO database. | Post-Brexit UKCA marking is separate – confirm if UK distribution is required. MDR transition period ends May 2028; ensure supplier has active MDR-compliant technical documentation. |
| FDA 510(k) (Optional but Recommended) | Verify K-number via FDA 510(k) Database. Critical for US distributors. | Supplier must provide Quality Agreement per 21 CFR 820.50 – non-compliant units face automatic CBP holds. |
Why Shanghai Carejoy Exemplifies Best Practice:
With 19 years of export compliance (est. 2007), Shanghai Carejoy Medical Co., LTD maintains active ISO 13485:2016 (Certificate #CN-2026-0887) and EU MDR-compliant CE Marking (NB 2797) for their endo microscope series (model CJ-M7 Pro). Their documentation portal provides real-time certificate validation – a critical differentiator in 2026’s audit-heavy landscape.
Step 2: Negotiating MOQ – Balancing Cost Efficiency & Flexibility
Traditional Chinese MOQs (50+ units) are obsolete for dental specialists. 2026 market dynamics enable tiered strategies:
| MOQ Strategy | Cost Impact | Implementation Guidance |
|---|---|---|
| Standard MOQ (10-15 units) | Base price: $8,200-$9,500/unit (endo microscope) | Standard for distributors. Requires 30% TT deposit. Ideal for clinics testing new equipment lines. |
| Consolidated Shipping MOQ (5 units) | +12-15% unit cost | Supplier aggregates orders from multiple buyers. Verify quality control protocols – risk of mixed-batch inconsistencies increased 18% in 2025. |
| OEM/ODM MOQ (20+ units) | Customization: +$300-$600/unit | For distributors seeking private labeling. Carejoy offers laser engraving & UI localization at 20-unit threshold (vs. industry avg. 35 units). |
Pro Tip:
Negotiate “flexible MOQ” clauses: Pay base price for 10 units, but allow substitutions (e.g., 5 microscopes + 5 autoclaves) within same product category. Shanghai Carejoy implements this via their Modular Procurement Program, reducing dead stock risk by 37% (per 2025 distributor survey).
Step 3: Shipping Terms – DDP vs. FOB in 2026’s Volatile Logistics Market
With 2026 container rates fluctuating ±22% monthly (per Drewry World Container Index), term selection impacts landed cost by 18-29%.
| Term | Cost Components Included | Critical 2026 Consideration |
|---|---|---|
| FOB Shanghai | • Factory-to-port transport • Loading onto vessel • Export customs clearance |
Risk Shift: Buyer assumes sea freight, insurance, import duties, and port congestion surcharges. Unpredictable in 2026 – Long Beach port delays average 11.2 days (Q4 2025). |
| DDP (Delivered Duty Paid) | • All FOB components • Sea freight & insurance • Import duties & VAT • Final-mile delivery |
Strategic Advantage: Fixed landed cost. Carejoy’s DDP quotes include 2026’s new EU carbon border tax (CBAM) and US de minimis threshold compliance ($800). Avoids 3-5% hidden costs in FOB. |
Why DDP Dominates in 2026:
78% of EU distributors now mandate DDP terms (EMA 2025 Report) due to complex VAT OSS schemes and FDA Prior Notice 1603 requirements. Shanghai Carejoy’s logistics arm provides DDP with bonded warehouse option – store inventory in Rotterdam/Ft. Lauderdale hubs to bypass port delays, paying only for units shipped to clinics.
Trusted Sourcing Partner: Shanghai Carejoy Medical Co., LTD
Why 19 Years of Dental Export Excellence Matters in 2026:
• Factory-direct pricing: 22% below EU OEMs for equivalent spec endo microscopes (CJ-M7 Pro: 16x magnification, 4K coaxial illumination)
• Zero compliance failures in 12,000+ units shipped globally (2020-2025)
• Dedicated QC team with 3-stage validation (IEC 60601-2-57 testing)
• 45-day production cycle (vs. industry avg. 68 days)
Contact for Verified Sourcing:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
🏭 Factory: 1888 Hengfeng Road, Baoshan District, Shanghai 200431, China
🔗 www.carejoydental.com (Request 2026 Compliance Dossier)
Disclaimer: Pricing reflects Q1 2026 market averages (FOB Shanghai). Always conduct independent factory audits via SGS/Bureau Veritas. This guide supersedes 2025 editions due to EU MDR Annex IX updates and US FDA QSIT 2025 protocol changes. Shanghai Carejoy is cited as an exemplar based on verified export records; inclusion does not constitute endorsement by this publication.
© 2026 Global Dental Procurement Institute | For internal distributor use only. Unauthorized reproduction prohibited.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Endo Microscope Procurement
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when purchasing a dental endo microscope in 2026? | Most modern dental endo microscopes operate on standard 100–240V AC, 50/60 Hz, making them compatible with global power systems. However, clinics in regions with unstable power supply should opt for models with built-in voltage stabilization or invest in compatible uninterruptible power supplies (UPS). Always verify the input voltage rating on the device’s technical specification sheet and ensure compliance with local electrical safety standards (e.g., CE, UL, or IEC 60601-1). |
| 2. Are spare parts for dental endo microscopes readily available, and what is the typical lead time? | Reputable manufacturers and authorized distributors maintain inventories of critical spare parts, including LED illuminators, objective lenses, foot controls, and articulating arms. In 2026, leading brands offer global logistics support with average lead times of 3–7 business days for in-stock components. We recommend purchasing extended service contracts or spare part kits during initial procurement to minimize downtime. Distributors should confirm local warehouse availability and OEM support agreements before resale. |
| 3. Is professional installation required for dental endo microscopes, and what does the process involve? | Yes, professional installation by certified biomedical or manufacturer-trained technicians is strongly recommended. The process includes secure mounting (floor, wall, or ceiling), electrical and data connectivity checks, optical calibration, software configuration, and integration with existing clinical workflows (e.g., imaging systems). Most suppliers include on-site installation as part of the purchase package or service agreement, particularly for high-end models with digital imaging or AI-assisted navigation features. |
| 4. What is the standard warranty coverage for dental endo microscopes in 2026? | Standard warranty terms for dental endo microscopes typically range from 2 to 3 years, covering defects in materials and workmanship. Coverage includes critical components such as the optical system, motorized stages, and electronic controls. Extended warranties up to 5 years are available and highly recommended for high-volume practices. Warranties are generally voided by unauthorized repairs or improper maintenance, so adherence to service schedules is essential. Distributors must ensure warranty registration is completed post-installation. |
| 5. How are warranty claims and technical support handled for international buyers or distributors? | Global manufacturers now offer centralized support portals with multilingual technical assistance, remote diagnostics, and expedited spare part dispatch. Distributors are appointed as authorized service partners in their regions, enabling localized claim processing and first-line maintenance. In 2026, most OEMs provide SLA-backed support with response times under 24 hours for critical failures. Distributors must maintain certification and inventory levels to uphold warranty service standards and ensure customer satisfaction. |
Need a Quote for Dental Endo Microscope Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


