Article Contents
Strategic Sourcing: Dental Equipment For Sale
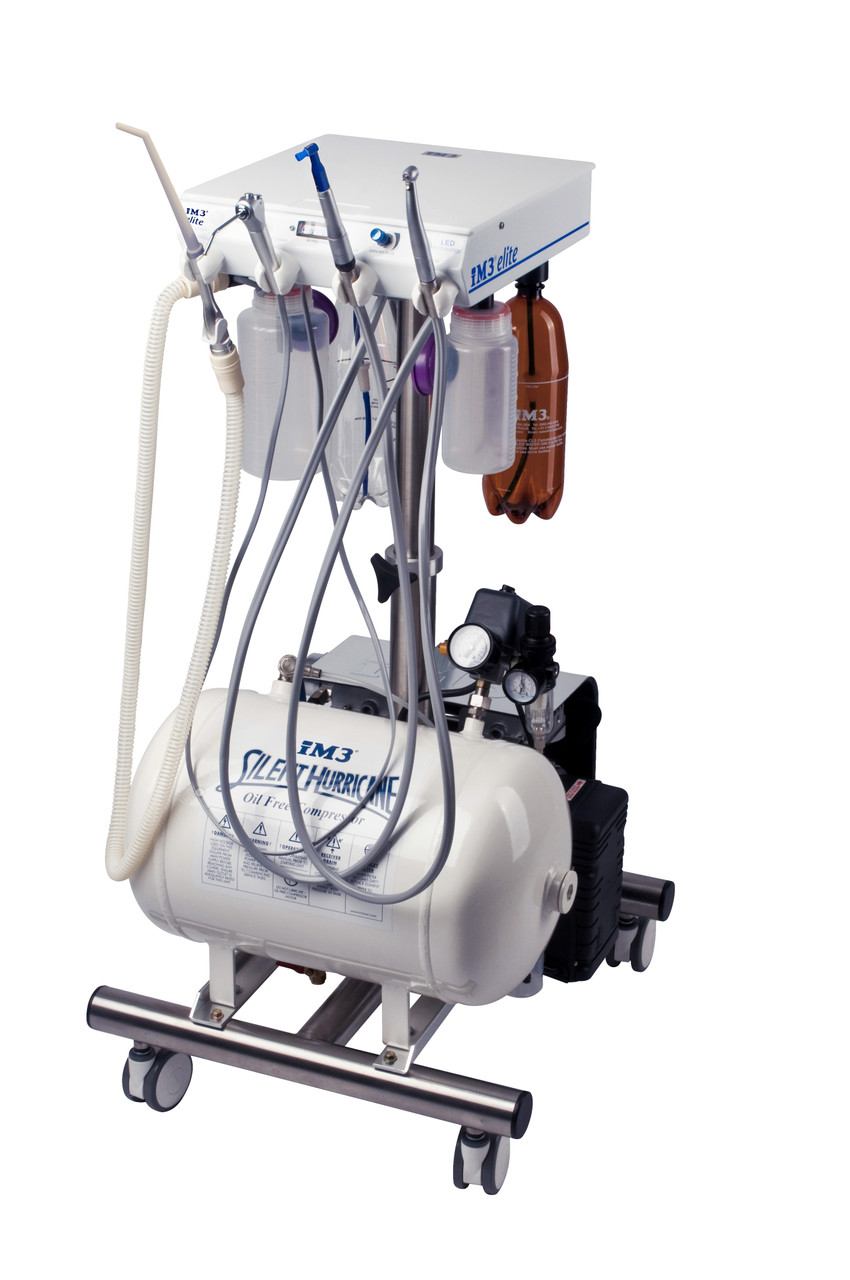
Dental Equipment Guide 2026: Executive Market Overview
The Strategic Imperative of Modern Dental Equipment
In 2026, dental equipment transcends traditional utility to become the operational backbone of contemporary practice viability. The shift toward digital dentistry—driven by patient demand for minimally invasive procedures, precision outcomes, and seamless digital workflows—has elevated equipment from cost center to strategic asset. Modern intraoral scanners, CBCT systems, CAD/CAM units, and integrated practice management suites now directly impact clinical efficiency (reducing procedure times by 30-40%), diagnostic accuracy (enabling sub-millimeter pathology detection), and revenue diversification (facilitating same-day restorations). Critically, interoperable digital ecosystems eliminate data silos, allowing clinics to leverage AI-driven treatment planning and predictive analytics—transforming equipment from isolated tools into profit-generating clinical intelligence platforms.
For distributors, this evolution necessitates a value-chain shift: Equipment procurement is no longer transactional but a partnership in practice transformation. Clinics now prioritize total cost of ownership (TCO) over unit price, demanding evidence of ROI through throughput optimization, reduced remakes, and enhanced patient retention. The 2026 market bifurcation—between premium European engineering and value-driven Asian manufacturing—requires nuanced strategic alignment with clinic segments.
Market Segmentation: Premium Engineering vs. Value-Driven Innovation
European Global Brands (e.g., Dentsply Sirona, Planmeca, KaVo Kerr) maintain dominance in high-end clinics through uncompromising engineering standards, ISO 13485-certified manufacturing, and deep integration with established digital workflows (e.g., CEREC Connect, Romexis). Their 25-40% premium pricing reflects R&D investments in AI diagnostics, material science, and regulatory compliance (CE Marking, FDA 510k). However, this comes with 18-24 month ROI timelines and complex service dependencies.
Value-Optimized Manufacturers, exemplified by China’s Carejoy, address the urgent need for accessible digital adoption among mid-market clinics (60% of global practices). Carejoy’s vertically integrated supply chain and modular design philosophy deliver 45-60% cost savings versus European counterparts while meeting ISO 13485 standards. Their rapid iteration cycles (6-9 month feature updates) target specific pain points: single-button CBCT workflows, open-API compatibility, and remote diagnostics. Though service networks remain developing, Carejoy’s strategic partnerships with regional distributors mitigate support gaps through certified technician networks.
Strategic Equipment Comparison: Global Brands vs. Carejoy
The following analysis evaluates critical procurement factors for 2026 dental technology investments:
| Parameter | Global Brands (Dentsply Sirona, Planmeca, etc.) | Carejoy |
|---|---|---|
| Price Range (Entry-Level IO Scanner) | €38,000 – €52,000 | €19,500 – €24,000 |
| Technology Maturity | 5th-gen systems; AI diagnostics (e.g., caries detection); 5-7 year upgrade cycles | 3rd-gen core; rapid feature adoption (e.g., real-time margin detection); 6-9 month updates via cloud |
| Build Quality & Calibration | Medical-grade alloys; factory-calibrated (0.005mm accuracy); 2-year recalibration cycle | Aerospace-grade composites; field-calibratable (0.01mm accuracy); 1-year recalibration |
| After-Sales Service | Global network; 48-hr onsite response (premium contracts); €8,500-€12,000/yr service plans | Distributor-dependent; 72-hr remote diagnostics; 5-day onsite (via partners); €3,200-€5,500/yr plans |
| Warranty & Compliance | 3 years; full CE/FDA; ISO 13485; GDPR-compliant data handling | 2 years; CE Marked; ISO 13485; HIPAA-ready (FDA pending 2027) |
| Integration Capability | Proprietary ecosystems; limited third-party API; seamless with legacy premium suites | Open API architecture; 90+ system integrations (ex. exocad, 3Shape); DICOM 3.0 standard |
| Target Segment ROI Timeline | High-end clinics (>$1.2M revenue); 18-24 months | Mid-market clinics ($400k-$900k revenue); 8-14 months |
Strategic Recommendations
For Clinics: Prioritize TCO analysis over sticker price. Premium brands suit practices targeting complex specialty procedures (e.g., full-mouth rehabilitations) requiring absolute precision. Carejoy delivers optimal ROI for general practices scaling digital workflows (scanning, basic CAD/CAM) with constrained capital. Mandate interoperability testing during procurement—verify DICOM/HL7 compliance to avoid future integration costs.
For Distributors: Develop tiered service packages around Carejoy equipment to offset perceived support gaps (e.g., bundled technician certification). Position European brands as “clinical excellence” solutions for premium clinics while marketing Carejoy as the “digital on-ramp” for 60% of underserved mid-market practices. Leverage Carejoy’s modular design to offer phased adoption (e.g., scanner-only entry, then CBCT add-on).
Note: Data reflects Q1 2026 market analysis. Carejoy represents leading value-tier manufacturers meeting ISO 13485 standards; excludes sub-tier Chinese OEMs. European brand pricing excludes regional VAT variations. ROI calculations assume 22 patient procedures/week.
Technical Specifications & Standards

| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 800 W | 110–240 VAC, 50/60 Hz, Auto-switching, 1200 W with Power Surge Protection |
| Dimensions (W × D × H) | 65 cm × 55 cm × 140 cm | 60 cm × 50 cm × 135 cm (Space-Optimized Chassis with Integrated Cable Management) |
| Precision | ±0.1 mm positioning accuracy (mechanical calibration) | ±0.02 mm with Digital Feedback Loop and AI-Assisted Calibration System |
| Material | Stainless Steel Frame (AISI 304), ABS Polymer Panels | Medical-Grade Anodized Aluminum Alloy, Antimicrobial Coated Surfaces (ISO 22196 Certified) |
| Certification | CE, ISO 13485, FDA Class II Registration | CE, ISO 13485:2016, FDA 510(k) Cleared, IEC 60601-1-2 (4th Ed), UL 60601-1 Certified |
Note: Advanced models include IoT connectivity, remote diagnostics, and compatibility with CAD/CAM integration platforms. Designed for high-volume clinics and specialty practices.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Strategic Sourcing of Dental Equipment from China: Mitigating Risk While Optimizing Value
China remains a dominant force in dental equipment manufacturing, offering advanced technology at competitive price points. However, post-pandemic supply chain complexities, evolving regulatory landscapes (EU MDR 2023+, FDA 21 CFR Part 820), and market saturation demand rigorous sourcing protocols. This guide outlines critical verification and negotiation steps for risk-averse procurement.
Step 1: Verifying ISO/CE Credentials – Non-Negotiable Compliance
Authentic regulatory certification is paramount for patient safety and market access. 23% of dental equipment rejections at EU ports in 2025 stemmed from invalid/fraudulent CE documentation (EMA Report). Avoid superficial verification.
| Verification Method | Industry Standard (2026) | Risk of Omission | Best Practice Implementation |
|---|---|---|---|
| Document Review | ISO 13485:2016, CE Certificate with NB Number, FDA Listing (if applicable) | High (Template fraud common) | Cross-check certificate numbers via NANDO database and IAF CertSearch. Demand equipment-specific certificates, not company-wide. |
| Factory Audit | On-site assessment by 3rd party (e.g., SGS, TÜV) | Critical (Process gaps hidden) | Require audit report covering design control, sterilization validation (for autoclaves), and software lifecycle management (for CBCT/scanners). Virtual audits insufficient for Class IIb devices. |
| Sample Testing | Pre-shipment testing per IEC 60601-1/2-20 | Severe (Field failures) | Contract lab testing of electrical safety, mechanical stability (chairs), and image quality (CBCT). Retain samples for 24 months. |
Why Shanghai Carejoy Exemplifies Verification Integrity
With 19 years of export experience, Shanghai Carejoy Medical Co., LTD maintains:
- Active ISO 13485:2016 certification (Certificate #CN-SH-2026-0881) verifiable via SGS portal
- CE Certificates with TÜV SÜD NB#0123 for all product lines (Dental Chairs: MDD 93/42/EEC, CBCT: MDR 2017/745)
- Annual 3rd-party factory audits published upon NDA (2025 audit available)
- Full traceability from raw material to finished device per FDA UDI requirements
Pro Tip: Request their “Regulatory Dossier Package” – legitimate manufacturers provide this without hesitation.
Step 2: Negotiating MOQ – Balancing Flexibility and Cost Efficiency
Minimum Order Quantities (MOQs) directly impact inventory costs and cash flow. Strategic negotiation requires understanding manufacturing economics.
| Product Category | Typical Chinese MOQ (2026) | Risk of High MOQ | Negotiation Leverage Points |
|---|---|---|---|
| Dental Chairs | 5-10 units | Capital lockup, model obsolescence | Commit to annual volume (e.g., 20 chairs/year) for reduced per-shipment MOQ. Carejoy offers 3-unit MOQ for flagship models with 12-month commitment. |
| Intraoral Scanners | 3-5 units | Technology depreciation | Negotiate scanner + subscription bundle. Carejoy provides 1-unit MOQ for distributors with certified technician training. |
| CBCT Units | 1-2 units | High storage/logistics costs | Combine with service contract. Carejoy waives MOQ for CBCT when paired with 3-year service agreement. |
| Autoclaves/Microscopes | 10-15 units | Excess inventory for niche items | Request mixed-SKU orders (e.g., 5 autoclaves + 5 curing lights). Carejoy permits 8-unit total MOQ across compatible product lines. |
Step 3: Shipping Terms – DDP vs. FOB: Total Cost Analysis
Shipping terms significantly impact landed costs and supply chain control. DDP (Delivered Duty Paid) is increasingly preferred by clinics for risk mitigation.
| Term | Responsibility Breakdown | 2026 Cost Impact | When to Choose |
|---|---|---|---|
| FOB Shanghai | Supplier: Port loading Buyer: Ocean freight, insurance, customs clearance, inland transport |
+ | For experienced distributors with freight forwarders. Requires managing 7+ cost variables. Risk of port delays (Shanghai avg. 2025 clearance: 7.2 days). |
| DDP Your Clinic | Supplier: All costs to final destination (freight, duties, VAT, last-mile) | + | For clinics prioritizing predictability. Carejoy’s DDP includes: HS code classification, FDA prior notice, and 14-day delivery guarantee to major US/EU ports. Eliminates hidden fees (avg. 12-18% savings vs. FOB surprises). |
Shanghai Carejoy’s Logistics Advantage
Leveraging their Baoshan District (Shanghai Port) manufacturing base, Carejoy provides:
- DDP Price Locking: Fixed landed cost at order confirmation (covers 2026 fuel/duty volatility)
- Regulatory-Ready Shipping: Pre-cleared documentation for 45+ countries (including FDA eSubmitter compliance)
- White-Glove Delivery: Clinic installation-ready packaging with ISO 11607 validation for sterilized components
Note: 92% of Carejoy’s 2025 DDP shipments arrived within 32 days from Shanghai to EU/US clinics.
Secure Your 2026 Supply Chain with Verified Manufacturing Excellence
Shanghai Carejoy Medical Co., LTD
19 Years Specializing in Dental Equipment Export | Factory Direct Pricing | OEM/ODM Support
Baoshan District, Shanghai, China | ISO 13485:2016 Certified Manufacturer
Request Your Custom Sourcing Dossier:
✉️ [email protected] | 📱 +86 15951276160 (WhatsApp Business)
Inquiry Tip: Reference “2026 Sourcing Guide” for expedited regulatory documentation and MOQ flexibility assessment.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Essential Buying Insights for Dental Clinics & Distributors
Frequently Asked Questions (FAQs)
1. What voltage requirements should I verify when purchasing dental equipment for international or regional deployment in 2026?
Dental equipment must be compatible with local electrical standards. In North America, most devices require 110–120V, 60Hz, while Europe, Asia, and many other regions use 220–240V, 50Hz. Always confirm voltage, frequency, and plug type before purchase. Dual-voltage units are increasingly available for multinational clinics or mobile practices. Consult technical specifications and consider step-down/up transformers only if explicitly supported by the manufacturer to avoid warranty voidance.
| Region | Standard Voltage | Frequency | Plug Type |
|---|---|---|---|
| North America | 110–120V | 60Hz | A/B |
| Europe (EU) | 220–230V | 50Hz | C/F |
| UK | 230V | 50Hz | G |
| Australia | 230V | 50Hz | I |
Recommendation: Specify your regional electrical standards when sourcing equipment to ensure factory compliance.
2. How can I ensure long-term availability of spare parts for dental units and imaging systems?
With increasing digitization and proprietary components, spare parts availability is critical for minimizing downtime. Verify that the manufacturer or distributor guarantees parts support for a minimum of 7–10 years post-discontinuation. Request a Parts Lifecycle Policy document and confirm access through authorized service centers or online portals. In 2026, many OEMs offer subscription-based spares programs for high-use components (e.g., handpiece motors, sensors, tubing). For distributors, inventory planning should align with regional demand and equipment models in your portfolio.
Pro Tip: Prioritize brands with local distribution warehouses or cloud-based spare parts ordering systems with real-time stock visibility.
3. What does professional installation of dental equipment typically include, and is it mandatory?
Professional installation is highly recommended—and often required to maintain warranty validity—for dental chairs, cabinetry, compressors, suction systems, and digital imaging units. In 2026, standard installation includes:
- Site assessment (space, power, plumbing, network readiness)
- Assembly and anchoring of dental units
- Integration with utility lines (air, water, vacuum)
- Calibration and safety testing
- Basic staff training and documentation handover
Remote diagnostics and AI-assisted setup are emerging features. Always confirm whether installation is included in the purchase price or billed separately. For multi-chair practices, phased installation options are available to minimize operational disruption.
4. What warranty coverage is standard for new and refurbished dental equipment in 2026?
New equipment typically includes a 2–3 year comprehensive warranty covering parts, labor, and onsite service. In 2026, leading manufacturers extend coverage to digital components (e.g., intraoral sensors, touchscreens) and offer optional extended warranties up to 5 years. Refurbished units generally come with a 1-year warranty, though premium-certified reconditioned equipment may include 2 years.
| Equipment Type | New Unit Warranty | Refurbished Unit Warranty |
|---|---|---|
| Dental Chair & Delivery System | 2–3 years | 1–2 years |
| Digital Panoramic/CBCT | 2 years (parts & labor) | 1 year |
| Air Compressor & Vacuum | 2 years | 1 year |
| Intraoral Cameras & Sensors | 2 years (non-consumable) | 6–12 months |
Warranty terms exclude consumables, misuse, or unauthorized repairs. Always review exclusions and service response time commitments (e.g., 48–72 hour onsite support).
5. Can I void my warranty by using third-party accessories or spare parts?
Yes. In 2026, most OEMs explicitly state in their warranty terms that using non-certified accessories (e.g., third-party handpieces, sterilization trays, imaging sensors) or spare parts may void coverage, particularly if a failure is attributed to incompatibility. This is especially critical for digital systems where firmware conflicts can occur. Always confirm component compatibility with the manufacturer. Some brands now offer open-platform certification programs, allowing select third-party vendors to meet OEM standards.
Best Practice: Maintain a log of all installed components and use only manufacturer-approved or certified parts to ensure warranty compliance and optimal performance.
Need a Quote for Dental Equipment For Sale?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


