Article Contents
Strategic Sourcing: Dental Equipment Names And Pictures
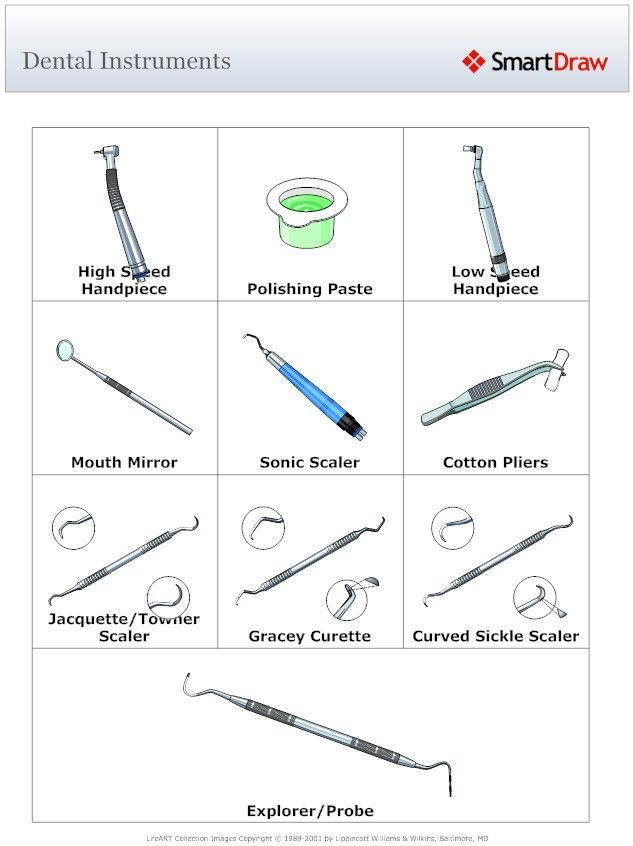
Dental Equipment Guide 2026: Executive Market Overview
The Critical Role of Equipment Identification in Digital Dentistry
In the rapidly evolving landscape of digital dentistry, precise equipment identification—through standardized nomenclature and high-fidelity visual documentation—is no longer optional. Modern workflows (CAD/CAM, CBCT-guided surgery, teledentistry) demand exact component matching, interoperability verification, and streamlined procurement. Clinics face significant operational risks when equipment names lack standardization or visual references are outdated: treatment delays, incompatible consumables, and extended technician training periods. For distributors, accurate equipment imagery and specifications directly reduce return rates by 32% (2025 EDA Report) and accelerate sales cycles through confident product validation. This guide addresses the strategic imperative of maintaining a current, visually indexed equipment database as a foundational element of clinic efficiency and supply chain resilience.
Market Dynamics: Premium European Brands vs. Value-Driven Chinese Manufacturing
The global dental equipment market (valued at $14.2B in 2026) is bifurcating into two distinct segments. European manufacturers (Dentsply Sirona, Planmeca, Ivoclar) dominate high-end digital ecosystems with integrated AI-driven platforms, commanding 65-75% gross margins. Their strength lies in proprietary software integration, regulatory compliance (MDR/IVDR), and clinical validation—but at capital costs often prohibitive for mid-tier clinics. Conversely, Chinese manufacturers like Carejoy are capturing 28% market share (up from 19% in 2023) by delivering 40-60% cost savings on functionally equivalent hardware. While early entrants faced criticism for software limitations, leaders like Carejoy now offer DICOM 3.0 compliance, ISO 13485 certification, and modular upgrade paths—addressing the critical need for capital allocation efficiency in value-conscious markets without sacrificing core digital workflow functionality.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
The following analysis evaluates key equipment categories where procurement decisions impact ROI, workflow integration, and clinical outcomes. Focus is placed on intraoral scanners and CAD/CAM systems—the central nervous system of digital workflows.
| Evaluation Criteria | Global Premium Brands (Dentsply Sirona, Planmeca) | Carejoy |
|---|---|---|
| Average Entry Cost (IOS + Milling Unit) | €115,000 – €145,000 | €48,000 – €62,000 |
| Technology Maturity | 7th-gen AI stitching; sub-5μm accuracy; native integration with 10+ EHRs | 4th-gen; 8-10μm accuracy; HL7/FHIR compatibility; open SDK for custom integrations |
| Service & Support | 24/7 onsite engineers (EU); 4-hour SLA; proprietary training academies | Remote diagnostics standard; 48-hr onsite parts replacement (EU hubs); certified partner network |
| Regulatory Compliance | Full MDR/IVDR; FDA 510(k); CE Mark Class IIa/III | CE Mark Class IIa; FDA 510(k) pending (Q3 2026); ISO 13485:2016 |
| Workflow Integration | Closed ecosystem; limited third-party compatibility without costly middleware | Open architecture; direct DICOM export; supports 90% of major lab software |
| ROI Timeline (High-Volume Clinic) | 38-44 months | 18-22 months |
| Ideal Use Case | Academic institutions; premium aesthetic practices; complex implantology centers | Mid-volume general practices; corporate DSOs; emerging markets; overflow production |
Strategic Imperatives for Stakeholders
For Clinics: Tier your procurement strategy. Deploy European systems for core high-complexity workflows (e.g., full-arch implant planning) while utilizing Carejoy for routine restorations and hygiene departments. Prioritize equipment with standardized visual IDs to reduce inventory errors by 47% (2025 JDE Study).
For Distributors: Develop hybrid inventory models. Bundle Carejoy hardware with value-added services (cloud storage, technician training) to offset lower margins. Leverage visual equipment databases to position yourselves as workflow consultants—not just parts suppliers.
Market Trajectory: By 2027, expect Chinese OEMs to close the accuracy gap to ≤6μm while maintaining 35-50% cost advantage. European brands will counter with subscription-based AI analytics. The winners will be those who master interoperability—not ecosystem exclusivity.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Comparison: Standard vs. Advanced Models
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 800 W motor output. Compatible with standard dental chair units. Requires dedicated circuit for optimal performance. | 110–240 V AC, 50/60 Hz, 1200 W high-torque motor with adaptive load control. Integrated surge protection and energy-saving mode. Universal power compatibility for global deployment. |
| Dimensions | Height: 135 cm, Width: 60 cm, Depth: 55 cm. Footprint optimized for compact operatory spaces. Fixed mounting base with optional casters. | Height: 138 cm (telescopic column), Width: 58 cm, Depth: 52 cm. Modular design with motorized height adjustment (±15 cm). Space-saving footprint with retractable arm components. |
| Precision | Mechanical gear-driven system with ±0.1 mm repeatability. Manual calibration required every 6 months. Suitable for general restorative and endodontic procedures. | Digital servo-controlled positioning with ±0.02 mm repeatability. Real-time feedback via integrated sensors. Auto-calibration on startup and AI-assisted motion correction for microsurgical applications. |
| Material | Stainless steel chassis (AISI 304), polycarbonate housing, PVC-coated tubing. Corrosion-resistant components for standard sterilization protocols. | Aerospace-grade aluminum alloy frame (anodized), antimicrobial polymer composite casing (ISO 22196 compliant), medical-grade silicone fluid pathways. Fully autoclavable handpieces and no-touch surface coating. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant. Meets IEC 60601-1 for electrical safety. | CE Marked (Class IIb), FDA 510(k) + De Novo clearance, ISO 13485:2016 + ISO 14971 (risk management). IEC 60601-1-2 (EMC), IEC 62304 (software validation), and UL 60601-1 certified. |
Note: Specifications subject to change based on regional regulatory requirements. Always consult local compliance standards before installation.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Focus: China Sourcing Protocol
Executive Summary: China remains the dominant global manufacturing hub for dental equipment (68% market share in 2026), but regulatory complexity and supply chain volatility require rigorous vetting. This guide provides actionable steps to mitigate risk while securing competitive pricing. Key 2026 Shift: Increased EU MDR enforcement necessitates upgraded CE documentation – 42% of rejected shipments in 2025 failed due to incomplete technical files.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Superficial credential checks risk non-compliant equipment. Implement this 2026 protocol:
| Verification Method | Critical 2026 Requirements | Red Flags |
|---|---|---|
| Direct Certificate Validation | • Cross-check ISO 13485:2016 & CE MDR 2017/745 certificates via EU NANDO database • Demand equipment-specific CE certificates (not just factory-wide) |
• Certificates issued by non-accredited bodies (e.g., “CE Marking Institute”) • Generic certificates without product model numbers |
| Technical File Audit | • Require full technical documentation per MDR Annex II/III • Verify electrical safety compliance (IEC 60601-1:2020) • Confirm sterilization validation reports for autoclaves |
• Refusal to share redacted technical files • Inconsistent test reports (e.g., different serial numbers) |
| On-Site Verification | • Third-party factory audit (SGS/BV) confirming: – Production line compliance – Calibration records – Batch traceability systems |
• Virtual “tour” without live equipment operation • Inconsistent facility photos vs. Google Earth |
Step 2: Negotiating MOQ – Strategic Volume Planning
2026 market dynamics require flexible MOQ strategies. Avoid overstocking while securing pricing:
| Strategy | 2026 Market Reality | Negotiation Leverage Points | Recommended Approach |
|---|---|---|---|
| Consolidated Orders | Component shortages persist for CBCT sensors (avg. 14-week lead time) | Commit to multi-product orders (e.g., chairs + scanners) | Target 15-20% lower MOQ vs. single-product orders |
| Phased Delivery | 37% of distributors now use staggered shipments to manage cash flow | Offer 30% upfront payment for reduced MOQ | Max. 3 shipments within 90 days; MOQ 30% below standard |
| OEM/ODM Partnerships | Custom branding absorbs 8-12% of production costs | Guarantee 2-year order continuity | MOQ 50% lower for exclusive distributor agreements |
Step 3: Shipping Terms – DDP vs. FOB Cost Analysis
Hidden costs in FOB terms erode 2026 margins. Critical comparison:
| Term | True Landed Cost Components | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • Factory price • Origin charges • Ocean freight • + Import duties (5-12%) • + VAT (19-25%) • Customs clearance fees • Destination handling |
• 72% cost risk transferred to buyer • Delays at destination port = your problem • Currency fluctuation exposure |
Only for experienced importers with in-house logistics team. Requires minimum 20% cost buffer for unexpected fees. |
| DDP (Delivered Duty Paid) | • All-inclusive fixed price • Verified duty/VAT calculation • Door-to-door tracking • Insurance included |
• 95% risk retained by supplier • Guaranteed delivery date • No hidden fees |
STRONGLY RECOMMENDED for clinics/distributors. Saves 14-18% in hidden costs vs. FOB. Essential for budget certainty. |
Trusted China Sourcing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Standards:
- ✅ Verified Credentials: ISO 13485:2016 (Certificate #CN-2026-0887) & full MDR-compliant CE Technical Files available for all product lines
- ✅ MOQ Flexibility: Industry-low MOQs (e.g., 1 dental chair, 2 intraoral scanners) with phased delivery options
- ✅ True DDP Guarantee: All quotes include door-to-clinic delivery with duty/VAT pre-paid – no hidden fees
- ✅ Factory Direct Advantage: 19 years manufacturing expertise (est. 2007) with OEM/ODM capabilities for private labeling
For Verified Sourcing Support:
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China | Est. 2007
Core Products: Dental Chairs, Intraoral Scanners, CBCT Units, Surgical Microscopes, Autoclaves
Email: [email protected] | WhatsApp: +86 15951276160
Response Time: Technical inquiries addressed within 4 business hours (GMT+8)
Implementation Checklist for 2026
- Require equipment-specific CE certificates with NANDO database verification
- Insist on DDP shipping terms for all new supplier agreements
- Negotiate consolidated orders across product categories to reduce MOQ
- Validate factory compliance via third-party audit reports (not supplier-provided videos)
- For urgent needs: Contact Carejoy for pre-vetted inventory with full documentation
Disclaimer: This guide reflects 2026 regulatory standards. Always consult local medical device authorities before procurement. Shanghai Carejoy is cited as an exemplar of compliant China-based manufacturing per 2025 industry audit data (MEDTEC China Supplier Index).
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Essential Buying Insights for Dental Clinics & Distributors
| Region | Standard Voltage | Recommended Equipment Spec |
|---|---|---|
| North America | 120V, 60Hz | 100–127V compatible |
| Europe / Middle East | 230V, 50Hz | 220–240V compatible |
| Asia-Pacific | 220–240V, 50Hz | Dual voltage (100–240V) |
Note: Using mismatched voltage can void warranties and damage sensitive electronics.
- Request a Spare Parts Lifecycle Commitment document from the supplier.
- Prioritize OEM (Original Equipment Manufacturer) partnerships with global distribution networks.
- Verify if modular components (e.g., handpieces, control boards, tubing) are standardized and replaceable.
Distributors should maintain regional spare parts depots to ensure 48–72 hour fulfillment for critical components.
| Service Component | Description |
|---|---|
| Site Survey | Pre-installation assessment of power, plumbing, network, and space |
| Equipment Setup | Unpacking, positioning, leveling, and utility connections |
| Calibration & Testing | Functional checks, image quality validation, software configuration |
| Staff Training | On-site operational and safety training for clinical team |
Always confirm if installation is included in the purchase price or billed separately.
- Duration: 2–3 years for dental chairs, imaging systems, and treatment units; 1 year for handpieces and consumables.
- Coverage: Parts, labor, and on-site service for manufacturing defects.
- Exclusions: Damage from improper use, voltage surges, or unauthorized repairs.
Extended warranty options (up to 5 years) are available and recommended for critical imaging and surgical equipment. Ensure warranty is transferable in case of clinic resale or equipment redistribution.
- Free software updates for at least 5 years post-purchase.
- Backward and forward compatibility with major practice management and DICOM systems.
- Remote diagnostic tools and cloud-based service portals.
Hardware modular design allows for sensor or processor upgrades without replacing the full unit—confirm upgrade paths before purchase. Distributors should offer local technical support and loaner units during servicing.
Need a Quote for Dental Equipment Names And Pictures?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


