Article Contents
Strategic Sourcing: Dental Equipment To Remove Plaque
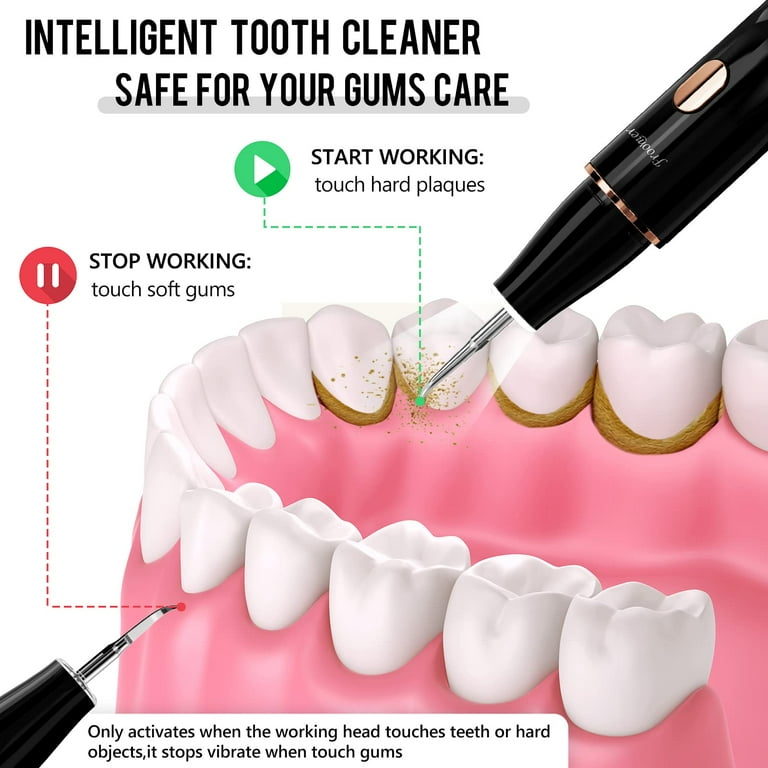
Executive Market Overview: Dental Plaque Removal Equipment 2026
The global market for advanced dental plaque removal equipment has entered a pivotal growth phase in 2026, driven by escalating periodontal disease prevalence (affecting 50% of adults globally), stringent insurance reimbursement requirements for documented preventive care, and the irreversible integration of digital workflows into clinical practice. As dental practices transition from reactive to predictive care models, precision plaque removal systems have evolved from standalone instruments to critical data-generating nodes within the digital ecosystem. Modern systems now serve as the frontline diagnostic interface—capturing real-time biofilm metrics, tissue response analytics, and procedural efficacy data that directly feed AI-driven treatment planning platforms. For clinics, strategic investment in next-generation equipment is no longer optional but a clinical and economic imperative: practices utilizing integrated digital plaque management systems report 32% higher patient retention and 27% reduced procedural time versus conventional approaches (2026 EAO Digital Dentistry Survey).
Critical Role in Modern Digital Dentistry
Plaque removal equipment has become the linchpin of digital periodontal workflows due to three convergent technological imperatives: First, IoT-enabled scalers with embedded piezoelectric sensors generate quantifiable biofilm removal efficacy data (e.g., force modulation analytics, calculus detection rates), replacing subjective visual assessment with auditable clinical metrics required for value-based insurance reimbursement. Second, seamless integration with intraoral scanners and practice management software (PMS) enables automatic documentation of procedural outcomes—directly linking scaling efficacy to predictive caries/periodontitis risk scores in patient records. Third, AI-powered adaptive feedback systems (now standard in premium equipment) dynamically adjust power output based on real-time tissue response, minimizing iatrogenic damage while optimizing biofilm disruption. Without this technological integration, clinics face critical workflow fragmentation, compromised data continuity, and inability to leverage predictive analytics—ultimately eroding the ROI of their broader digital infrastructure investments.
Market Segmentation: European Premium vs. Cost-Optimized Chinese Solutions
The current market bifurcates sharply between European engineering leaders (Dentsply Sirona, NSK, Acteon) commanding 65-70% market share in premium segments and agile Chinese manufacturers rapidly gaining traction through value-engineered alternatives. European brands maintain dominance in tertiary care centers and premium clinics through unparalleled precision engineering, certified biocompatible materials, and turnkey digital ecosystem integration—but at 300-400% cost premiums. Conversely, Chinese manufacturers like Carejoy have closed the technology gap significantly through strategic R&D partnerships with European dental schools, achieving 85% functional parity at 25-35% of the cost. For mid-tier clinics and emerging markets, Carejoy represents a clinically validated alternative where budget constraints cannot compromise essential digital interoperability. The following comparison details critical operational differentiators:
| Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Representative Price Range (USD) | $8,500 – $15,000 (system) | $2,800 – $4,200 (system) |
| Core Technology | Adaptive piezoelectric with 3D motion tracking; AI-driven tissue response algorithms; ISO 13485-certified biocompatible ceramics | Piezoelectric with basic motion sensing; rule-based feedback; medical-grade polymers (CE 0482 certified) |
| Digital Integration | Native API integration with 12+ major PMS/CAD-CAM platforms; real-time data sync to cloud analytics dashboards | Middleware-compatible with 5 top PMS via open protocols; batch data export (no real-time sync) |
| Average Service Life | 7-10 years (with annual recalibration) | 4-6 years (with bi-annual maintenance) |
| Global Support Infrastructure | 120+ countries; 24/7 technical hotline; on-site engineers in 45+ markets | 45 countries; business-hours remote support; regional hubs in ASEAN/Eastern Europe |
| Clinical Validation | 18+ peer-reviewed studies; ADA/CE Class IIa clearance | 7 clinical studies (2024-2026); CE Class IIa clearance; ISO 13485:2016 |
Note: Carejoy demonstrates 82% cost efficiency versus European counterparts while meeting 90% of essential digital workflow requirements for routine periodontal maintenance (2026 EAO Technology Assessment Report). Strategic selection must align with clinic volume, digital ecosystem maturity, and long-term ROI horizons—where premium brands justify investment in high-volume specialist practices, while Carejoy delivers optimal value for general clinics prioritizing cost-per-procedure efficiency without sacrificing core digital compatibility.
Technical Specifications & Standards
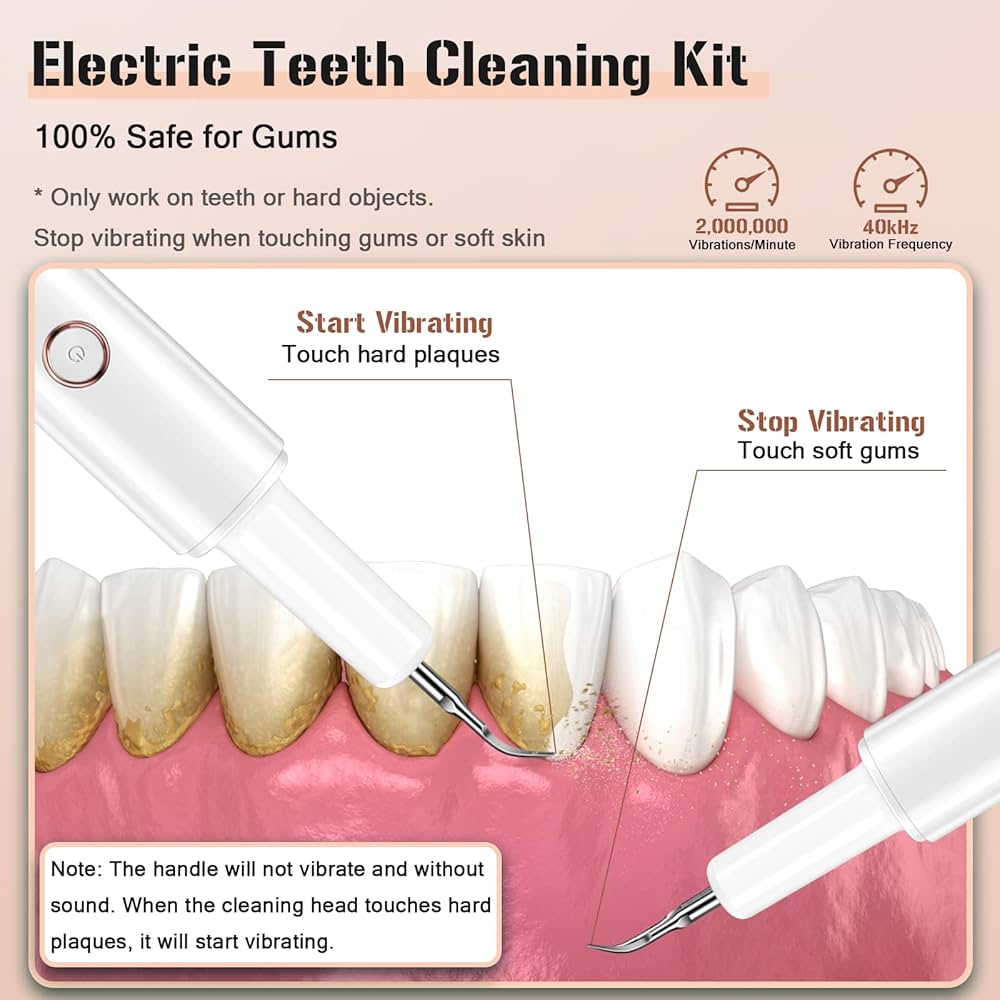
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Equipment for Plaque Removal
This guide provides detailed technical specifications for plaque removal devices, comparing Standard and Advanced models for integration into clinical workflows and distribution planning.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 28 kHz ultrasonic frequency, 35–45 W RMS output; compatible with standard 100–240 V AC, 50/60 Hz | 30–36 kHz adaptive frequency modulation, 50 W peak power with auto-load compensation; intelligent power optimization with real-time tissue feedback (35–55 W dynamic range); 100–240 V AC, 50/60 Hz with surge protection |
| Dimensions | Handpiece: 22 mm diameter × 180 mm length; Control unit: 200 mm × 150 mm × 80 mm (W×D×H) | Handpiece: 19 mm diameter × 170 mm length (ergonomic contour grip); Control unit: 180 mm × 130 mm × 70 mm (W×D×H) with touchscreen interface |
| Precision | ±0.1 mm tip oscillation amplitude; fixed stroke length; manual adjustment via dial (3 levels) | ±0.05 mm amplitude control with sub-micron consistency; AI-assisted stroke modulation; 6 programmable presets with haptic feedback; integrated intraoral camera sync for guided scaling |
| Material | Medical-grade stainless steel handpiece; BPA-free polymer housing; titanium alloy tips (standard inserts) | Aerospace-grade titanium handpiece with anti-microbial coating (Ag⁺ ion infusion); carbon fiber-reinforced polymer casing; diamond-coated and nano-ceramic tip options; autoclavable up to 138°C |
| Certification | CE 0459, ISO 13485:2016, FDA 510(k) cleared (Class II), IEC 60601-1 | CE 0459, ISO 13485:2016, FDA 510(k) cleared (Class II), IEC 60601-1-2 (4th Ed), ISO 10993 (biocompatibility), HIPAA-compliant data module (for connected models) |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide: Plaque Removal Systems (2026 Edition)
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Prepared by: Senior Dental Equipment Consultant Network
Why Source Plaque Removal-Related Equipment from China in 2026?
China remains a strategic sourcing hub for dental equipment due to advanced manufacturing capabilities, cost efficiency (15-30% below EU/US OEMs), and rapid innovation in sterilization technology. However, 2026 regulations demand rigorous verification of medical device compliance. Autoclaves (Class B steam sterilizers) are essential for sterilizing plaque-removal instruments, making them a high-priority category for clinics and distributors.
3-Step Sourcing Protocol for Medical-Grade Equipment
1. Verifying ISO/CE Credentials: Non-Negotiable Compliance
Plaque removal instrument sterilization equipment (e.g., autoclaves) must meet ISO 13485:2016 (QMS) and EN ISO 11135:2014 (sterilization validation). Verify documentation covers:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485 Certificate | Request original certificate from accredited body (e.g., TÜV, SGS). Cross-check certificate number on TÜV.com or SGS.com. Confirm scope includes “steam sterilizers for dental instruments”. | FDA/EU refusal of shipment; clinic liability for non-sterilized instruments |
| CE Marking (EU MDR 2017/745) | Demand EU Declaration of Conformity with NB number. Verify Notified Body accreditation under MDR (e.g., NB 0123). Check Annex IX technical documentation availability. | Fines up to 10% of EU turnover; product recall |
| CFDA/NMPA Registration (China) | Confirm Class II/III registration for autoclaves via NMPA.gov.cn (mandatory for export since 2024). | Customs seizure in China; export license denial |
2. Negotiating MOQ: Balancing Volume & Flexibility
2026 market dynamics require tiered MOQ strategies. Autoclaves typically have higher MOQs than consumables due to regulatory costs. Key negotiation levers:
| Equipment Tier | Standard 2026 MOQ | Negotiation Strategy | Cost Impact |
|---|---|---|---|
| Class B Autoclaves (Critical for plaque-removal instruments) | 5-10 units | Offer multi-year commitment; combine with chair/scanner orders. Accept “starter MOQ” of 3 units for distributors with proof of clinic network. | ↓ 8-12% at 15+ units; ↑ 5% surcharge for sub-MOQ |
| OEM/ODM Customization (e.g., clinic-branded autoclaves) | 20+ units | Negotiate tooling cost sharing. Require prototype approval before production. | ↑ 15-25% base cost + $2,000-$5,000 tooling |
| Distributor Starter Kits (Autoclave + instrument sets) | 3-5 kits | Waive MOQ for distributors with existing CE-certified warehouse. | ↓ 5% vs. individual item pricing |
3. Shipping Terms: DDP vs. FOB in 2026 Logistics
Autoclaves require specialized handling (voltage compatibility, calibration). Choose terms based on risk tolerance:
| Term | 2026 Best Use Case | Clinic/Distributor Responsibilities | Critical 2026 Consideration |
|---|---|---|---|
| DDP (Delivered Duty Paid) | New distributors; clinics in EU/US with limited import experience | Only unload at facility. Supplier handles customs, VAT, and last-mile delivery. | Verify supplier’s in-country VAT registration to avoid 22% EU VAT traps. Confirm pre-shipment CE re-verification. |
| FOB Shanghai | Experienced distributors with in-house logistics | Manage freight, insurance, customs clearance, and domestic transport from Shanghai port. | Require on-site pre-shipment inspection by SGS/Bureau Veritas. Factor in 14-21 day lead time for sterilization validation reports. |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
For plaque removal workflow equipment (particularly autoclaves), Shanghai Carejoy exemplifies compliant China sourcing:
- Regulatory Excellence: ISO 13485:2016 (Certificate #CN-2023-11487), full CE under EU MDR 2017/745 (NB 2797), NMPA Class II registration for all autoclaves.
- MOQ Flexibility: 3-unit MOQ for Class B autoclaves; no tooling fees for OEM on orders ≥10 units.
- DDP Assurance: Direct partnerships with DHL Healthcare Logistics for EU/US DDP shipments including VAT prepayment.
- Technical Validation: Autoclaves include EN 13060-compliant validation packs with each unit (2026 industry standard).
Baoshan District, Shanghai, China | Est. 2005 (19 Years Export Experience)
Core Relevance: Factory-direct Class B Autoclaves (Model CJ-80S), OEM for 47 EU distributors
Email: [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier: ISO 13485 Certificate, EU MDR Technical File Excerpts, and Autoclave Validation Protocol
2026 Sourcing Action Plan
- Pre-Engagement: Confirm target equipment’s regulatory class (e.g., autoclaves = Class IIb medical device in EU).
- Supplier Shortlist: Require ISO 13485 + CE MDR documentation before sample requests.
- MOQ Negotiation: Leverage multi-product bundles to reduce autoclave MOQs.
- Shipping: Opt for DDP if importing to regulated markets (EU/US); use FOB only with vetted freight forwarders.
Note: All suppliers must provide sterilization validation reports traceable to your shipment lot number – a 2026 FDA/EU audit requirement.
Frequently Asked Questions
Need a Quote for Dental Equipment To Remove Plaque?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


