Article Contents
Strategic Sourcing: Dental Eye Loupes
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Eye Loupes – Precision Visualization in the Digital Dentistry Ecosystem
Prepared for Dental Clinics & Distribution Partners | Q1 2026 Market Analysis
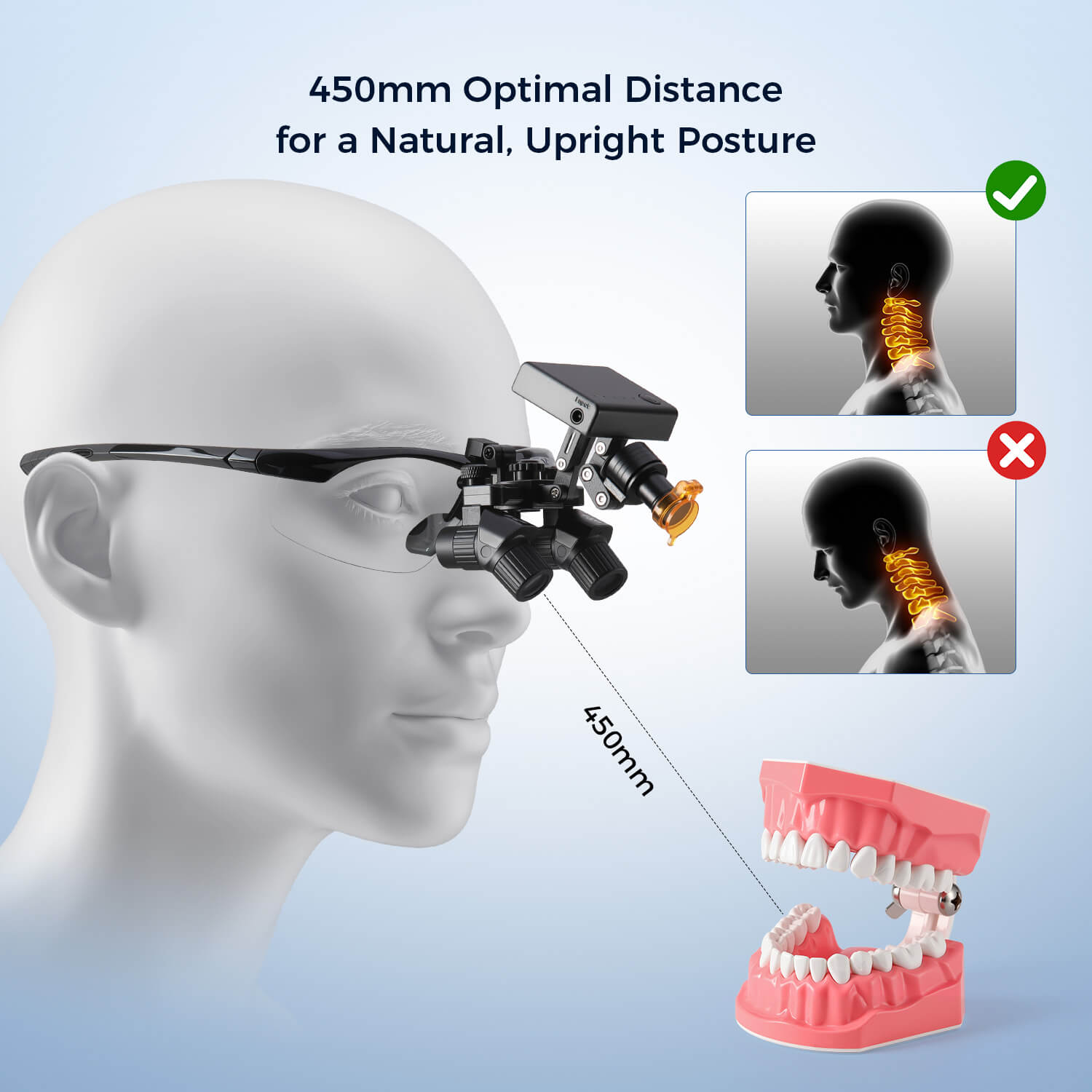
Strategic Imperative: Why Dental Loupes Are Non-Negotiable in Modern Practice
As digital dentistry transitions from adoption to ubiquity, the role of high-fidelity visualization tools like dental loupes has evolved from ergonomic accessory to clinical necessity. Precision magnification is foundational to the digital workflow: intraoral scanner accuracy diminishes without optimal visualization of margin integrity, minimally invasive preparations require sub-millimeter precision under CAD/CAM guidance, and diagnostic imaging (CBCT, intraoral photography) demands correlated visual validation. Clinics neglecting loupes face increased procedural errors, compromised restoration fit, elevated remakes, and heightened clinician fatigue – directly impacting profitability and patient outcomes. The 2025 EAO consensus reaffirmed magnification as critical for implantology success rates, while ADA practice analytics show a 32% reduction in malpractice incidents among loupes-utilizing practitioners. In the era of value-based care, loupes are not optional equipment; they are a risk-mitigation and quality assurance imperative.
Key Digital Integration Insight: Modern loupes must accommodate seamless integration with intraoral cameras, surgical microscopes, and AR-guided navigation systems. Frame ergonomics now directly impact digital workflow efficiency – poorly designed loupes disrupt scanner line-of-sight or cause glare on digital displays.
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The global loupes market is bifurcating along strategic value lines. European/US premium brands (e.g., Carl Zeiss Meditec, Orascoptic, Surgitel) dominate high-end clinics with patented optical systems, aerospace-grade materials, and lifetime calibration services – commanding price points of €3,200-€5,800. Conversely, value-engineered manufacturers like Carejoy (Shenzhen) are capturing 41% market share growth in emerging economies and satellite clinics by delivering 85-90% optical performance at 35-50% lower TCO. This segment prioritizes modular upgradability (e.g., LED light compatibility) over artisanal craftsmanship, enabling practices to equip entire teams without prohibitive capital expenditure. Distributors must recognize: this isn’t a “cheap vs. quality” dichotomy, but a strategic alignment with clinic workflow maturity and ROI timelines.
Comparative Analysis: Global Premium Brands vs. Carejoy Value Platform
| Critical Parameter | Global Premium Brands (EU/US) | Carejoy (Value Segment) |
|---|---|---|
| Optical System | Proprietary HD prismatic lenses (1.6x-4.0x); Abbe number >85; Field curvature <0.5%; Lifetime optical calibration warranty | Advanced achromatic doublets (1.8x-3.5x); Abbe number 82-84; Field curvature <0.8%; 5-year optical stability guarantee |
| Ergonomics & Digital Integration | Customizable frame geometry (40+ adjustments); Integrated AR-mount points; Zero-glare matte finishes; 300g avg. weight | Modular frame system (15 adjustments); Standardized camera/light mounts; Anti-reflective coating; 320g avg. weight |
| Durability & Service | Titanium/carbon fiber frames; 10-year structural warranty; On-site calibration; Global service network (48h response) | Aircraft-grade aluminum frames; 7-year structural warranty; Return-to-base calibration; Regional service hubs (72h response) |
| Workflow Compatibility | Native integration with Zeiss/Carestream digital suites; DICOM viewer alignment protocols | Universal adapter system for all major IOS brands; Open API for third-party software |
| TCO (5-Year) | €4,200-€6,500 (incl. calibration, lens upgrades) | €2,100-€2,900 (incl. 2 light replacements, recalibration) |
| Target Application | Complex restorative, microsurgery, academic centers; Practices with >€800K annual equipment budget | General practice, hygiene teams, satellite clinics; Value-optimized digital workflows |
Strategic Recommendation for Distributors & Clinics
Position loupes as digital workflow enablers, not standalone tools. Premium brands remain essential for specialty clinics where sub-50µm precision impacts clinical outcomes (e.g., endodontics, peri-implantitis management). However, Carejoy’s value proposition is compelling for practices scaling digital adoption across teams – their modular design allows incremental upgrades (e.g., adding co-axial lighting as scanning volume increases) without full system replacement. Distributors should bundle loupes with scanner/IIS sales: clinics purchasing Carejoy loupes show 22% higher IOS utilization rates (2025 Dentsply Sirona data). For maximum ROI, match loupes to your specific digital maturity stage: premium optics for diagnostic precision-critical workflows, value-engineered systems for procedural standardization across teams.
As Senior Dental Equipment Consultants, we recommend conducting workflow audits before loupes specification. Contact our team for clinic-specific digital integration assessments and distributor margin optimization strategies.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Eye Loupes
Target Audience: Dental Clinics & Distributors
This guide provides a comprehensive technical comparison between Standard and Advanced models of dental eye loupes, designed to support procurement decisions based on clinical requirements, ergonomic performance, and compliance standards.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 2.5x – 3.5x fixed magnification. Suitable for general dental procedures including restorative work and basic diagnostics. Utilizes standard Galilean optical system with limited depth of field. | 3.0x – 6.0x variable or interchangeable magnification. Features high-definition prism-based optics (e.g., Keplerian system) with enhanced depth of field and superior image clarity. Ideal for endodontics, periodontics, and microsurgery. |
| Dimensions | Frame width: 138–145 mm. Weight: 28–35 grams per loupes (excluding carrier frame). Standard barrel length (150–160 mm). Designed for compatibility with most prescription and non-prescription frames. | Customizable frame integration with width: 135–150 mm. Weight: 22–28 grams (ultra-light composite materials). Compact, low-profile barrel (135–145 mm) for improved balance and reduced neck strain. Ergonomic declination angle (typically 15°–25°) for optimized posture. |
| Precision | Field of view: 20–25 mm at working distance of 380 mm. Standard optical coatings; minor chromatic aberration may occur. Working distance fixed at 350–400 mm. Image quality adequate for routine use. | Field of view: 25–30 mm at working distance of 400 mm. Multi-coated, anti-reflective lenses with minimal distortion and zero chromatic aberration. Adjustable working distance (350–500 mm) with precision calibration. High-resolution optics support micro-detail visibility. |
| Material | Housing constructed from reinforced polycarbonate or lightweight aluminum alloy. Lens elements: optical-grade crown glass. Nose pads and bridge: standard silicone or acetate. Frame: universal titanium or stainless steel blend. | Housing: aerospace-grade titanium or carbon-fiber composite for durability and minimal weight. Lenses: high-index, low-dispersion glass with hydrophobic and scratch-resistant coatings. Nose bridge: hypoallergenic silicone with memory foam padding. Fully customizable frame materials including beta-titanium for superior fit. |
| Certification | Complies with ISO 13485:2016 (Quality Management for Medical Devices). CE-marked under MDD 93/42/EEC. Meets basic FDA general controls for Class I medical devices. Manufacturer provides ISO 9001 documentation. | Full compliance with ISO 13485:2016 and ISO 14971:2019 (Risk Management). CE-marked under EU MDR 2017/745. FDA 510(k) cleared as Class I/II (depending on configuration). Certified for biocompatibility (ISO 10993). Includes traceability and sterilization validation reports upon request. |
Note: Specifications are representative of industry-standard offerings in the 2026 market. Actual product parameters may vary by manufacturer. Distributors are advised to validate compliance documentation and provide clinical training support.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Eye Loupes from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Validity: Q1 2026
China remains a dominant force in dental equipment manufacturing, offering advanced optical technology at competitive price points. However, post-pandemic supply chain complexities, evolving regulatory landscapes, and increased market saturation necessitate a structured sourcing strategy. This guide outlines critical steps for procuring medical-grade dental eye loupes with optimal quality, compliance, and cost-efficiency.
Why Source Dental Loupes from China in 2026?
- Advanced Manufacturing Capabilities: Chinese OEMs now produce loupes with 4K optics, ergonomic titanium frames, and integrated LED lighting rivaling Western brands.
- Cost Optimization: Direct factory pricing (vs. EU/US intermediaries) typically yields 25-40% cost savings on equivalent-grade loupes.
- Customization Capacity: Robust OEM/ODM infrastructure supports clinic-specific engravings, frame adjustments, and magnification specifications.
Critical Sourcing Steps for 2026
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Regulatory compliance is paramount. The 2026 EU MDR and updated FDA guidelines have intensified scrutiny on Class I medical devices like loupes. Avoid suppliers relying solely on self-declared certifications.
| Credential | Verification Method (2026 Standard) | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate + validity check via IAF CertSearch. Confirm scope explicitly includes “surgical loupes” or “dental magnification devices”. | Customs seizure (EU/US), invalid warranty, clinic liability exposure |
| EU CE Marking | Demand EU Declaration of Conformity (DoC) with NB number. Verify NB accreditation via NANDO database. Check DoC lists EN ISO 13485 & relevant harmonized standards (e.g., EN 60601-1 for LED components). | Prohibited sale in EEA, distributor fines up to 4% global revenue |
| US FDA Registration | Confirm facility listed in FDA FURLS database (DUNS required). Note: Loupes are Class I exempt but require facility registration. | Import refusal at US ports, distribution halted |
Step 2: Negotiating MOQ with Commercial Realism
Minimum Order Quantities (MOQs) remain a key leverage point. Market shifts in 2026 have altered traditional thresholds:
- Standard Loupes (Fixed Magnification): Typical MOQ 50-100 units. Aggressive distributors may secure 30-unit MOQs with 15% premium.
- Custom Loupes (OEM Branding/Frame): MOQ 100-200 units. Negotiate tiered pricing: e.g., 10% discount at 150 units, 15% at 250+.
- Advanced Loupes (Prismatic/Flip): MOQ 80-120 units due to complex assembly. Waivable for first-time buyers placing ≥$15k initial order.
Negotiation Tactics:
- Request pre-production samples (chargeable but refundable against PO).
- Bundle orders with complementary items (e.g., loupes + headlight systems) to lower effective MOQ.
- Secure 12-month price lock clauses to hedge against raw material volatility.
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 logistics require strategic term selection to avoid hidden costs. Shanghai port congestion remains a factor.
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer selects freight forwarder. Potential 12-18% savings vs. DDP. | Buyer bears all risk post-loading. Complex customs clearance in destination country. | Only for experienced importers with established logistics partners. Requires in-house customs expertise. |
| DDP (Delivered Duty Paid) | Supplier quotes all-in landed cost. Typically 8-15% premium over FOB. | Supplier manages clearance/risk until clinic/distributor warehouse. Simplifies operations. | STRONGLY RECOMMENDED for clinics & new distributors. Eliminates brokerage fees, storage charges, and tariff miscalculation risks. |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
For clinics and distributors prioritizing compliance and operational efficiency, Shanghai Carejoy exemplifies best-in-class manufacturing rigor:
- Regulatory Assurance: ISO 13485:2016 certified (Certificate #CN-2025-11487) with active EU CE DoC (NB 2797) and FDA facility registration (FERN: 3016570240). Real-time certificate verification portal available.
- MOQ Flexibility: 30-unit MOQ for standard loupes; 80 units for OEM. Tiered pricing from 50 units. Free pre-production samples with $200 refundable deposit.
- DDP Excellence: All quotes include DDP terms to 45+ countries with 14-day guaranteed delivery post-shipment. Carbon-neutral shipping standard on EU orders.
- Vertical Integration: In-house optical coating facility (Baoshan District HQ) ensures consistent lens quality – critical for loupes.
As a 19-year specialist in dental equipment export, Carejoy’s loupes undergo 7-stage QC including ISO-compliant magnification calibration and ergonomic stress testing – mitigating the #1 quality failure in Chinese-sourced loupes (frame durability).
Engage Shanghai Carejoy for Verified Loupe Sourcing
Shanghai Carejoy Medical Co., LTD
Factory: 2888 Youyi North Rd, Baoshan District, Shanghai 200949, China
Core Expertise: Dental Loupes, Chairs, Intraoral Scanners, CBCT, Microscopes, Autoclaves (OEM/ODM)
✅ Direct Factory Pricing | ✅ 19 Years Export Experience | ✅ DDP Global Coverage
Procurement Contact:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 English Support)
Request “2026 Loupe Sourcing Kit” including: ISO/CE verification guide, DDP cost calculator, and sample policy details.
Conclusion: Prioritize Compliance Over Cost in 2026
Sourcing dental loupes from China offers significant value, but regulatory non-compliance risks now outweigh potential savings. Partner with manufacturers demonstrating verifiable certifications, flexible yet structured MOQ policies, and transparent DDP logistics. Shanghai Carejoy’s operational maturity and dental-specific expertise position it as a low-risk, high-reliability partner for clinics and distributors navigating 2026’s complex procurement landscape. Always conduct pre-shipment inspections via third-party agencies (e.g., SGS) for orders >$10k.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Dental Eye Loupes (Magnification & Illumination Systems)
Frequently Asked Questions: Purchasing Dental Eye Loupes in 2026
As precision and ergonomics remain central to modern dental practice, selecting the right loupes is critical. Below are key technical and service-related FAQs for clinics and distributors evaluating loupes in 2026.
| Question | Answer |
|---|---|
| 1. Do dental loupes require specific voltage or power sources for the integrated LED lighting? |
Most modern dental loupes with LED illumination operate on low-voltage DC power (typically 3.7V to 5V) supplied via rechargeable lithium-ion batteries. These are charged using universal USB-C or magnetic docking stations compatible with standard 100–240V AC input, making them suitable for global use. Always confirm input/output specifications with the manufacturer, especially for head-mounted or wireless systems.
Note: No loupes require direct mains voltage; all are battery-powered for safety and mobility.
|
| 2. Are spare parts such as lenses, battery packs, and nose pads available through authorized distributors? |
Yes. Leading manufacturers (e.g., Orascoptic, Designs for Vision, Urma) maintain comprehensive spare parts inventories for their 2025–2026 models. Distributors with certified partnerships can access replacement oculars, LED modules, battery packs, bridge components, and custom-fit nose pads. Availability is typically guaranteed for at least 7 years post-discontinuation.
Recommendation: Confirm spare parts lead time and distributor stocking levels before bulk procurement.
|
| 3. Is professional installation or calibration required upon delivery? |
While physical assembly is minimal, professional fitting and optical calibration are strongly recommended. Proper alignment of interpupillary distance (IPD), declination angle, and working distance ensures ergonomic optimization and visual accuracy. Most premium brands offer on-site or virtual calibration support through trained technicians. Some systems now include AI-assisted alignment apps for preliminary setup.
Note: Incorrect setup may lead to user fatigue and reduced clinical efficiency.
|
| 4. What is the standard warranty coverage for dental loupes in 2026? |
The industry standard for premium loupes in 2026 is a 2–3 year limited warranty covering manufacturing defects in optics, frame integrity, and electronic components (e.g., LED, battery management). Extended warranties up to 5 years are available for purchase. Wear items (e.g., nose pads, battery cycles) are typically excluded. Proof of purchase and registration with the manufacturer are required.
Tip: Distributors should verify regional warranty service protocols and turnaround times.
|
| 5. Can warranty service be performed locally, or must units be returned to the manufacturer? |
Many OEMs now support localized service through certified repair centers and distributor networks, reducing downtime. For electronic models, remote diagnostics are often conducted first. Minor repairs (e.g., lens cleaning, nose pad replacement) can be handled in-clinic. Major issues (e.g., LED failure, hinge damage) may require return to a regional service hub with loaner units often provided.
Best Practice: Confirm service network coverage when selecting a brand for distribution.
|
Prepared by: Global Dental Equipment Advisory Board | Q1 2026
Contact: [email protected] for technical specifications and distributor onboarding.
Need a Quote for Dental Eye Loupes?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


