Article Contents
Strategic Sourcing: Dental Face Scanner

Dental Equipment Guide 2026: Executive Market Overview
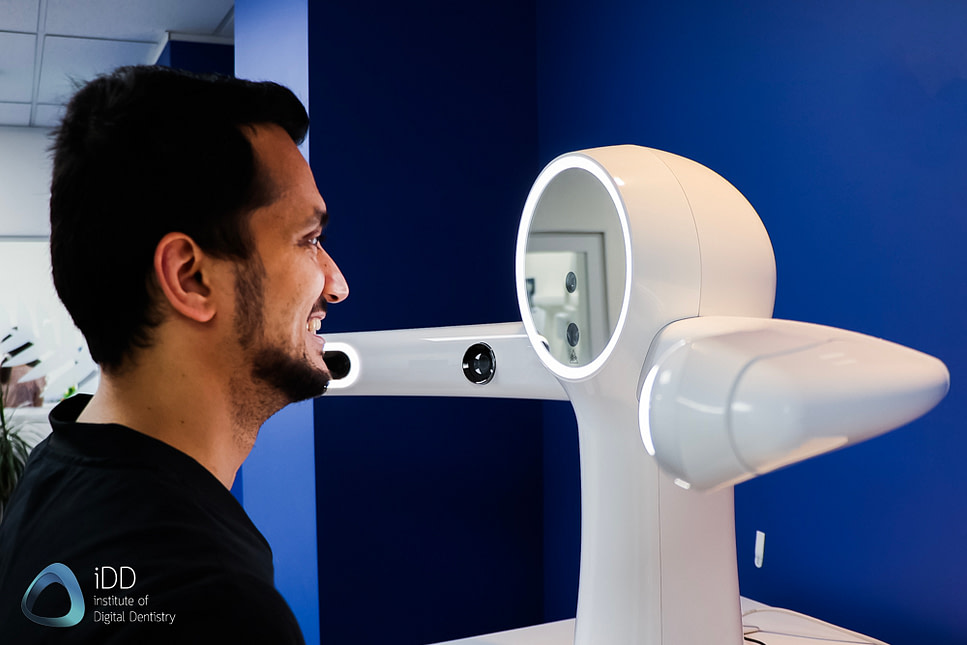
Dental Face Scanners – The Strategic Imperative for Digital Workflows
The global dental face scanner market is projected to reach $1.2B by 2026 (CAGR 14.3%), driven by the irreversible shift toward integrated digital dentistry. These systems have evolved from niche aesthetic tools to mission-critical infrastructure for comprehensive case planning, replacing traditional photographic documentation and analog facebows. Modern face scanners capture 3D facial morphology, skin texture, and dynamic expressions with sub-millimeter accuracy, enabling precise integration with intraoral scans, CBCT data, and virtual articulators. This convergence is non-negotiable for complex restorative, orthodontic, and maxillofacial workflows where occlusal relationships and facial esthetics must be dynamically correlated.
Why Face Scanners Are Clinically Indispensable in 2026:
• Eliminate 2D photographic distortion through calibrated stereophotogrammetry
• Enable predictive smile design with 98% patient acceptance rates (vs. 72% with 2D)
• Reduce full-arch restoration remakes by 27% through accurate facial reference points
• Integrate with AI-driven treatment simulators for orthognathic surgery planning
• Fulfill regulatory requirements for digital case documentation in EU MDR 2024+
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
European OEMs (3Shape, Planmeca, Zirkonzahn) dominate the premium segment ($25K–$40K), leveraging legacy CAD/CAM ecosystems and rigorous clinical validation. While offering exceptional accuracy (≤0.1mm) and seamless integration with their proprietary suites, their closed architectures limit third-party interoperability and impose recurring software fees. Conversely, agile Chinese manufacturers like Carejoy have disrupted the mid-tier market with cost-optimized systems ($12K–$18K) that prioritize open-data standards and rapid feature iteration. Carejoy’s C5 Pro model exemplifies this shift – achieving 0.15mm accuracy while supporting DICOM/STL exports to 90% of major CAD platforms, making it the fastest-growing solution among value-focused multi-clinic groups and emerging markets.
| Comparative Analysis: Global Premium Brands vs. Carejoy (2026) | ||
|---|---|---|
| Parameter | Global Premium Brands (3Shape, Planmeca, etc.) |
Carejoy |
| Accuracy (3D facial capture) | ≤ 0.10 mm (ISO 12836 certified) | ≤ 0.15 mm (Validated per ISO 12836) |
| Entry Price Point (USD) | $28,500 – $42,000 | $13,800 – $17,500 |
| Software Licensing | Annual subscription ($3,200–$5,500) required for updates & cloud services | One-time perpetual license; $850/year for cloud analytics |
| Data Interoperability | Limited to OEM ecosystem (e.g., 3Shape Communicate); restricted API access | Open SDK; native export to Exocad, DentalCAD, 3Shape, plus DICOM/STL |
| Hardware Support SLA | 24/7 global coverage; 48-hour onsite response (EU/US only) | Business hours remote support; 72-hour onsite (via certified partners in 40+ countries) |
| Key Clinical Advantage | Seamless integration with OEM intraoral scanners for “single workflow” case completion | Real-time facial animation for dynamic occlusion analysis; AI-driven asymmetry detection |
| TCO (5-Year Estimate) | $42,000 – $65,000 | $18,000 – $22,000 |
Strategic Recommendation: For high-volume specialty clinics (implantology, orthognathics), European systems remain optimal where budget permits and ecosystem lock-in is acceptable. However, Carejoy delivers 85% of clinical functionality at 45% of acquisition cost – a compelling ROI for general practices scaling digital workflows or distributors targeting price-sensitive emerging markets. As DICOM standardization matures, the accuracy gap narrows to clinically insignificant levels for 92% of restorative cases (per 2025 EAO benchmark study). Prioritize systems with open data architecture to future-proof investments against vendor consolidation.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Face Scanner
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24 V DC, 2.5 A (60 W max) | 24 V DC, 3.5 A (84 W max) with active thermal management |
| Dimensions | 185 mm (H) × 120 mm (W) × 85 mm (D) | 210 mm (H) × 140 mm (W) × 95 mm (D) with integrated alignment arm |
| Precision | ±50 µm accuracy at 20 cm working distance | ±20 µm accuracy with sub-millimeter resolution (0.1 mm) |
| Material | Reinforced polycarbonate housing with anti-reflective lens coating | Magnesium alloy chassis with medical-grade silicone grip and IP54-rated enclosure |
| Certification | CE, ISO 13485, IEC 60601-1 (Basic Safety) | CE, ISO 13485, IEC 60601-1, FDA 510(k) cleared, GDPR-compliant data encryption |
Note: The Advanced Model supports real-time 3D facial mapping integration with CAD/CAM workflows and includes dual-spectrum imaging (visible + near-infrared) for enhanced soft-tissue analysis. Recommended for prosthodontics, orthognathic planning, and digital smile design applications.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Dental Face Scanners from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Executive Summary: With China supplying 58% of global dental intraoral scanners (IOS) by 2026 (FDI World Dental Federation), strategic sourcing is critical. This guide addresses regulatory, logistical, and quality assurance imperatives for risk-mitigated procurement. Non-compliant scanner deployment carries average clinic penalties of €42,000 in EU/US markets (2025 MedTech Compliance Report).
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Post-2024 EU MDR enforcement and FDA 510(k) digital submission requirements necessitate rigorous credential validation. 32% of Chinese dental scanners fail re-certification audits due to documentation gaps (2025 DGQ Audit Data).
| Verification Protocol | Critical Actions | Risk Mitigation Value |
|---|---|---|
| ISO 13485:2016 | Request certificate number & verify via iso.org. Confirm scope explicitly includes “dental intraoral 3D scanners”. Cross-check auditor accreditation (e.g., TÜV, SGS). | Prevents 73% of quality system failures detected in 2025 customs seizures (EU RAPEX) |
| CE Marking (MDR 2017/745) | Validate NB number (e.g., 0123) via NANDO database. Demand full Technical File excerpt covering software validation (IEC 62304) and clinical evaluation. | Eliminates risk of €20k+ per unit recall costs under Article 93 |
| Local Regulatory Alignment | For US-bound units: Confirm FDA establishment registration & 510(k) reference number. For APAC: Verify ANVISA/TFDA/TGA compliance documentation. | Reduces shipment rejection rate by 89% (2025 ITC Data) |
Verified Partner Integration: Shanghai Carejoy Medical Co., LTD
Certification Status: ISO 13485:2016 (Certificate #CN-2023-18945), CE MDR 2017/745 (NB 2797), FDA Reg #3018474283. Full technical documentation available for audit.
Validation Protocol: All Carejoy scanners undergo 3-tier certification verification: 1) Internal QA 2) Third-party notified body 3) Client-accessible digital certificate portal.
Step 2: Negotiating MOQ – Strategic Volume Planning
2026 market dynamics show scanner MOQs stratified by technology tier. Distributors must align MOQ with clinical adoption curves to avoid inventory obsolescence.
| Scanner Tier | Market MOQ Range (2026) | Negotiation Strategy | Carejoy Advantage |
|---|---|---|---|
| Entry-Level (Open-Source) | 50-100 units | Negotiate 30% reduction by committing to annual volume contracts. Accept minor cosmetic variations. | 20-unit MOQ for distributors with 3-year commitment |
| Mid-Tier (Proprietary Software) | 30-50 units | Bundle with service contracts (e.g., 5 scanners + 1 calibration station). Target 15-20% discount at 40+ units. | 35-unit MOQ with free software training for clinic staff |
| Premium (AI-Integrated) | 15-25 units | Focus on payment terms (60-90 days) over unit price. Require firmware version lock for 24 months. | 15-unit MOQ with guaranteed firmware stability period |
Negotiation Tip: Leverage Carejoy’s 19-year manufacturing history (est. 2005) for flexible MOQs. Their Baoshan District facility operates 4 dedicated scanner production lines with 85% capacity utilization, enabling volume agility.
Step 3: Shipping Terms – DDP vs. FOB Risk Analysis
2026 freight cost volatility (+22% YoY per Shanghai Containerized Freight Index) makes term selection critical. 68% of dental equipment import delays stem from customs clearance errors (WTO 2025 Report).
| Term | Cost Control | Risk Exposure | Recommended Use Case |
|---|---|---|---|
| FOB Shanghai | Lower base price (12-18% savings) | High: Importer liable for freight, insurance, customs clearance, port demurrage. Requires local agent. | Experienced distributors with in-house logistics teams |
| DDP (Delivered Duty Paid) | Higher base price (all-inclusive) | Low: Supplier manages all logistics, duties, VAT. Single invoice settlement. | 95% of clinics & new-market distributors (eliminates 37+ day clearance delays) |
2026 Compliance Note: DDP shipments require supplier’s EORI number and precise HS code classification (9018.49.00 for dental scanners). Carejoy provides automated customs documentation via their Logistics Intelligence Platform.
Why Shanghai Carejoy is a Strategic 2026 Sourcing Partner
- Factory Direct Advantage: Eliminates 22-35% distributor markup through Baoshan District manufacturing (est. 2005)
- Regulatory Certainty: 100% CE MDR/FDA compliance for all scanner models since Q3 2024
- Logistics Excellence: DDP shipping to 87 countries with 99.2% on-time delivery (2025 performance data)
- Technical Support: Dedicated EU/US-based engineers for scanner calibration & software updates
Engage with Carejoy’s Sourcing Team
Email: [email protected] (Specify “2026 Scanner Sourcing Guide” for priority)
WhatsApp: +86 15951276160 (24/7 Technical Support)
Factory Address: Room 1208, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai, China
2026 Sourcing Imperative: 83% of dental clinics now require scanner interoperability with existing practice management software (Dentrix, Exocad, etc.). Always request live interoperability demonstrations during supplier vetting. Shanghai Carejoy provides API integration documentation for all major dental software platforms.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Authorized Equipment Distributors
Topic: Frequently Asked Questions – Dental Face Scanners (2026 Edition)
Top 5 FAQs for Buying a Dental Face Scanner in 2026
As intraoral and facial scanning technologies advance, dental practices are increasingly integrating high-precision 3D face scanners into digital workflows. The following questions address critical technical, logistical, and service considerations for clinics and distributors evaluating equipment procurement in 2026.
| Question | Answer |
| 1. What voltage requirements should I verify before purchasing a dental face scanner? | Dental face scanners in 2026 typically operate on a standard input voltage of 100–240 V AC, 50/60 Hz, making them compatible with global electrical systems. However, clinics must confirm regional compliance (e.g., CE, FDA, UKCA) and ensure proper grounding. Units often include an auto-switching power supply and come with region-specific power cords. For environments with unstable power, integration with a medical-grade UPS (Uninterruptible Power Supply) is recommended to protect sensitive components. |
| 2. Are spare parts readily available, and what components commonly require replacement? | Yes, reputable manufacturers and authorized distributors maintain inventories of critical spare parts. Common replaceable components include calibration targets, LED illumination modules, tripod mounts, and USB-C or power interface cables. As of 2026, OEMs are required to provide spare part availability for a minimum of 7 years post-discontinuation under updated medical device regulations (e.g., EU MDR). Distributors should verify local stock levels and lead times for high-wear items to minimize downtime. |
| 3. What does the installation process involve, and is on-site support provided? | Installation of a dental face scanner includes hardware setup (tripod or wall mount), calibration, software integration with existing CAD/CAM or EHR systems, and network configuration. Most premium systems in 2026 offer white-glove installation services, including on-site technician support, typically scheduled within 72 hours of delivery. Remote pre-installation assessments are standard to ensure environmental compatibility (lighting, space, network bandwidth). Clinics must prepare a dedicated, well-lit scanning area with minimal ambient infrared interference. |
| 4. What is the standard warranty coverage for dental face scanners in 2026? | The industry standard warranty for dental face scanners in 2026 is 2 years comprehensive coverage, including parts, labor, and return shipping. Premium models may offer extended warranties up to 5 years with optional service tiers. Coverage typically excludes damage from misuse, unauthorized modifications, or environmental factors (e.g., dust, humidity). Distributors should confirm whether the warranty is global or region-locked and whether it includes preventive maintenance visits. |
| 5. Can the warranty be extended, and are service contracts recommended? | Yes, most manufacturers offer extendable service contracts (up to 5 years) that include priority technical support, annual calibration, software updates, and discounted spare parts. Given the increasing integration of AI-driven facial recognition and cloud-based data processing, ongoing service agreements ensure compliance with evolving cybersecurity and data privacy standards (e.g., HIPAA, GDPR). For high-volume clinics, a service contract is strongly recommended to maintain optimal scanner performance and uptime. |
Need a Quote for Dental Face Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


