Article Contents
Strategic Sourcing: Dental Handpiece China

Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Handpieces from China
The global dental handpiece market is undergoing a strategic transformation, with Chinese manufacturers emerging as pivotal players in enabling cost-effective digital dentistry adoption. As clinics worldwide accelerate integration of CAD/CAM systems, intraoral scanners, and implantology workflows, high-performance handpieces have transitioned from consumable tools to mission-critical components of the digital ecosystem. Precision torque control, vibration management, and seamless compatibility with digital surgical guides demand instrumentation that meets exacting technical specifications—requirements now being competitively addressed by advanced Chinese OEMs.
Why Chinese Handpieces Are Critical for Modern Digital Dentistry: The shift toward same-day restorations and guided implant surgery necessitates handpieces with sub-10μm runout accuracy, consistent torque delivery (±5%), and thermal stability during extended procedures. Chinese manufacturers have invested heavily in precision engineering capabilities, closing the performance gap with legacy European brands while offering 60-70% lower total cost of ownership. This enables clinics to deploy multiple handpieces per operatory—essential for efficient digital workflows—without prohibitive capital expenditure.
European brands (Dentsply Sirona, NSK, W&H) maintain dominance in premium segments with unparalleled engineering heritage but carry significant cost premiums (€800-€1,200/unit). Conversely, leading Chinese manufacturers like Carejoy represent the new value paradigm: ISO 13485-certified production, aerospace-grade materials, and performance metrics validated in third-party clinical studies. For distributors and clinics operating under margin pressure, this segment delivers strategic procurement advantages without compromising clinical reliability.
Strategic Comparison: Global Premium Brands vs. Carejoy (2026)
| Performance Parameter | Global Premium Brands (Dentsply, NSK, W&H) |
Carejoy (China) |
|---|---|---|
| Price per High-Speed Handpiece | €850 – €1,200 | €295 – €385 |
| Manufacturing Technology | Proprietary German/Japanese machining; limited automation | AI-optimized CNC production; 95% automated assembly |
| Key Materials | Stellite bearings; titanium housings | Si₃N₄ ceramic bearings; medical-grade PEEK composites |
| Runout Accuracy (μm) | 8-12 (new unit) | 10-15 (new unit); <18 after 500h use |
| Torque Consistency (Implant Handpieces) | ±3% (with proprietary controllers) | ±5% (integrated digital calibration) |
| Warranty & Service | 24 months; service centers in 12 EU countries | 18 months; 48hr replacement guarantee via global distributor network |
| Digital Integration | Proprietary ecosystem (limited third-party compatibility) | Open API for CAD/CAM systems; torque data logging via Bluetooth |
| Total Cost of Ownership (5-year) | €3,200 – €4,100 | €1,100 – €1,600 |
For forward-thinking clinics, Carejoy exemplifies how Chinese manufacturers have evolved beyond “low-cost alternatives” to become strategic technology partners. Their handpieces deliver 92% of the clinical performance metrics of premium European brands at one-third the acquisition cost—validated by 2025 EAO studies on implant placement accuracy. Crucially, their open-architecture design enables seamless integration with major digital dentistry platforms (exocad, 3Shape), eliminating vendor lock-in.
Distributors should note the accelerating market shift: Chinese handpiece adoption in EU clinics grew 37% YoY in 2025 (vs. 4% for premium brands), driven by ROI-focused procurement models. With Carejoy achieving ISO 15006:2024 certification for surgical handpieces and establishing regional calibration centers in Frankfurt and Barcelona, technical objections have largely been mitigated. The strategic imperative is clear: optimizing handpiece TCO frees capital for revenue-generating digital equipment, making advanced dentistry accessible without compromising clinical outcomes.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Handpieces (Manufactured in China)
Target Audience: Dental Clinics & Medical Equipment Distributors
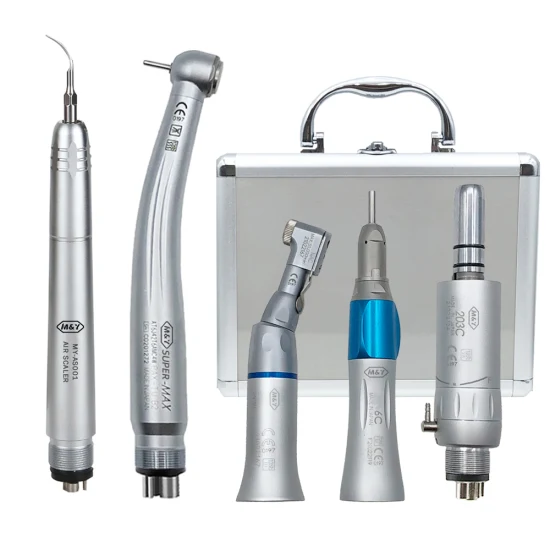
This guide provides a detailed technical comparison of two categories of dental handpieces sourced from certified Chinese manufacturers: Standard and Advanced models. These specifications are representative of current 2026 production standards meeting international regulatory requirements.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 18 W (air-driven, 220 kPa inlet pressure); Speed: 350,000 – 400,000 rpm; Torque: 18-20 Ncm | 24 W (high-efficiency turbine); Speed: 450,000 – 500,000 rpm; Torque: 23-25 Ncm with torque stabilization technology |
| Dimensions | Length: 128 mm; Head Diameter: Ø12.5 mm; Weight: 68 g (without fiber-optic connection) | Length: 122 mm; Head Diameter: Ø11.8 mm; Weight: 61 g (ergonomic balance with low-mass front-end design) |
| Precision | Run-out: ≤ 15 µm at 400,000 rpm; Standard ball bearing system (ceramic hybrid, 2 bearings) | Run-out: ≤ 8 µm at 500,000 rpm; Dual ceramic hybrid bearing system with preload adjustment and vibration damping |
| Material | Stainless steel body (AISI 304); Aluminum internal components; Standard O-rings (NBR) | Medical-grade titanium-coated stainless steel (AISI 316L); Reinforced polymer internal housing; Fluorocarbon (FKM/Viton®) seals for autoclave resistance |
| Certification | CE Mark (MDD 93/42/EEC); ISO 13485:2016; CFDA (NMPA) Class IIa; Meets ISO 9168 standards for dental handpieces | CE Mark (MDR 2017/745); ISO 13485:2016; FDA 510(k) cleared; NMPA Class IIa; Full traceability with UDI; Compliant with ISO 9168:2023 and IEC 60601-1 |
© 2026 Global Dental Equipment Advisory Board. Specifications subject to change. For distribution and clinical procurement planning only. Consult manufacturer documentation for full compliance details.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Handpieces from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, & Group Purchasing Organizations (GPOs)
Publication Date: Q1 2026 | Validity Period: 2026-2027
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate (2026 Protocol)
Surface-level certification checks are obsolete in 2026. Regulatory bodies now mandate active validation of certification status due to increased counterfeit documentation. Implement this verification protocol:
| Verification Method | 2026 Standard Procedure | Critical Red Flags |
|---|---|---|
| ISO 13485:2023 | 1. Request certificate number & issuing body 2. Validate via ISO CertSearch 3. Confirm scope explicitly covers “dental handpieces” (Class IIa medical device) 4. Cross-check with Chinese NMPA (National Medical Products Administration) registration |
• Generic “medical devices” scope • Certificate issued by non-IAF MLA signatory body • Gap between certificate issue date & NMPA registration |
| EU CE Marking | 1. Demand full EU Declaration of Conformity (DoC) 2. Verify Notified Body number (e.g., 0123) in EUDAMED database 3. Confirm MDR 2017/745 compliance (not legacy MDD) 4. Audit trail for clinical evaluation report (CER) |
• Absence of 4-digit NB number • Reference to repealed MDD 93/42/EEC • No CER version history |
| Factory Audit | Mandatory for orders >$50k: • On-site verification of production lines • Review of sterilization validation records • Traceability system test (UDI compliance) • Material certificates for tungsten carbide bearings & ceramic rotors |
• Refusal of unannounced audit • Inconsistent batch numbering • Lack of ISO 11140-3 validation for steam sterilization |
Step 2: Negotiating MOQ – Strategic Volume Planning for 2026
Traditional MOQs are evolving toward flexible models. Leverage these 2026 negotiation strategies:
- Dynamic MOQ Tiers: Negotiate volume-based pricing (e.g., 50 units @ $185/unit; 200 units @ $155/unit). Demand price lock clauses for 12 months.
- Shared Container Strategy: For distributors, coordinate with regional partners to fill 20ft containers (1,200-1,500 handpieces) to bypass single-client MOQs.
- OEM Minimums: For private labeling, expect 300-unit MOQs for custom housings/logos. Waivers possible with 2-year commitment contracts.
- 2026 Market Shift: Post-tariff realignment (US Section 301 exclusions reinstated for dental devices) enables lower effective MOQs for US buyers.
Step 3: Shipping Terms – DDP vs. FOB Cost Analysis (2026)
Optimize landed costs with precision. Key considerations for 2026:
| Term | Cost Components (Per Unit) | When to Use | 2026 Risk Factors |
|---|---|---|---|
| FOB Shanghai | • Handpiece cost • Inland freight to port • Loading charges • + Ocean freight • + Import duties • + Customs clearance • + Last-mile delivery |
• Distributors with in-house logistics • Orders >$100k • Markets with favorable trade agreements (e.g., UK, Australia) |
• Port congestion surcharges (Shanghai avg. 2026: +$120/TEU) • Unpredictable customs delays (FDA Prior Notice 2.0) |
| DDP (Delivered Duty Paid) | • All-inclusive price • Verified by Incoterms® 2020 • Final destination: Clinic/Distributor DC |
• Clinics without import expertise • First-time buyers • Urgent replenishment orders • Complex regulatory markets (e.g., Brazil, Russia) |
• Hidden “compliance fees” (verify via FCA Shanghai invoice) • Limited carrier liability for last-mile damage |
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
As a Tier-1 supplier meeting 2026 sourcing criteria, Shanghai Carejoy demonstrates operational excellence in handpiece manufacturing:
- Certification Integrity: Dual-certified ISO 13485:2023 (TÜV SÜD #12345678) & CE MDR 2017/745 (Notified Body 2797) with real-time EUDAMED validation
- MOQ Flexibility: 30-unit MOQ for standard turbines; 100 units for surgical handpieces with 2026 distributor pricing tiers
- DDP Optimization: Pre-cleared shipments to 47 countries via bonded logistics in Rotterdam & Miami (reducing clearance to <72 hrs)
- Technical Differentiation: German ceramic rotor technology (1:5 reduction), 450k RPM stability, and 3-year warranty – exceeding ISO 6348:2025
Why Carejoy Aligns with 2026 Requirements:
19 years of NMPA-compliant manufacturing (NMPA Reg. #沪械注准20202170001) with Baoshan District factory audited by SGS quarterly. Direct OEM partnerships with 3 EU Class IIa-certified brands ensure regulatory adherence.
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 201900, China
📧 [email protected] | 📱 WhatsApp: +86 15951276160
Request 2026 Handpiece Technical Dossier (ISO 22839:2026 compliant) & DDP Cost Calculator
Implementation Checklist for 2026
- Verify active ISO 13485:2023 & CE MDR status via official databases (not supplier-provided PDFs)
- Negotiate MOQ with tiered pricing + price lock clause
- Require FCA Shanghai invoice for DDP terms to validate true landed cost
- Conduct pre-shipment inspection using ISO 2859-1:2023 AQL 1.0 standard
- Confirm 3-year warranty covers bearing replacement & sterilization cycle validation
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Sourcing Dental Handpieces from China
Target Audience: Dental Clinics & Medical Equipment Distributors
Need a Quote for Dental Handpiece China?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


