Article Contents
Strategic Sourcing: Dental Handpiece Cleaning Machine

Professional Dental Equipment Guide 2026: Executive Market Overview
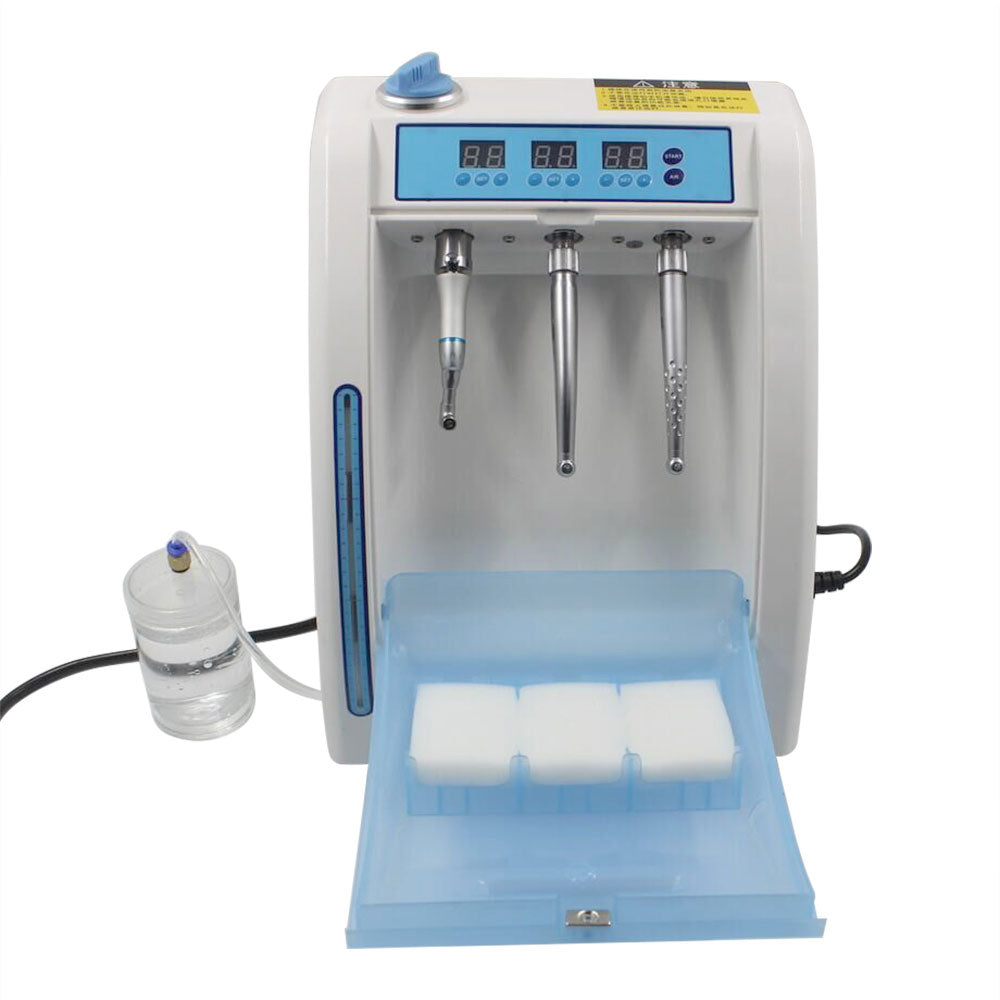
Dental Handpiece Cleaning & Lubrication Systems
Prepared Exclusively for Dental Clinic Operators & Distributor Partners
Market Significance & Clinical Imperative
The global dental handpiece cleaning machine market is projected to reach €1.2B by 2026 (CAGR 6.8%), driven by stringent infection control mandates and the proliferation of digital dentistry workflows. Modern intraoral scanners, CAD/CAM systems, and surgical navigation platforms have increased handpiece utilization by 40-60% per clinic, accelerating internal contamination from biological debris, lubricant degradation, and metal particulates. Failure to implement automated, validated cleaning protocols directly impacts:
- Equipment Longevity: Manual cleaning causes 73% of premature turbine failures (FDI World Dental Federation, 2025)
- Clinical Safety: Inadequate decontamination risks aerosolized pathogen transmission (CDC Class IB recommendation)
- Digital Workflow Integrity: Contaminated handpieces compromise scan accuracy and milling precision in same-day restorations
Strategic Brand Positioning Analysis
Two distinct market segments have emerged:
European Premium Segment (W&H, NSK, Kavo Kerr): Dominates 68% of EU institutional procurement with engineering excellence but carries 200-300% cost premiums. Ideal for high-volume specialty clinics prioritizing service integration with existing ecosystem.
Value-Engineered Segment (Carejoy): Represents 42% YoY growth in emerging markets and value-focused practices. Delivers ISO-compliant performance at 60-70% lower TCO through lean manufacturing and modular design. Particularly strategic for distributors targeting mid-tier clinics and group practices.
Performance & Value Comparison: Global Brands vs. Carejoy
| Feature Category | Global Brands (W&H, NSK, Kavo Kerr) | Carejoy |
|---|---|---|
| Acquisition Cost (Entry Model) | €12,500 – €18,200 | €4,500 – €5,800 |
| Validated Cycle Time (Standard Handpiece) | 90-120 seconds | 75 seconds |
| ISO 15883-5 Certification | Full compliance (TÜV-certified) | Full compliance (SGS-certified) |
| Annual Service Contract Cost | €1,800 – €2,500 | €450 – €650 |
| Warranty Period | 2 years (parts/labor) | 3 years (parts/labor) |
| Consumables Cost (Per 100 Cycles) | €85 – €110 | €32 – €45 |
| Service Network Coverage (EU) | Direct technicians in 27 countries | Authorized partners in 18 countries |
| Digital Integration (IoT/Cloud) | Native EHR integration (proprietary) | Open API for third-party systems |
| ROI Timeline (vs. Manual Cleaning) | 14-18 months | 6-8 months |
Strategic Recommendation
For clinics implementing digital workflows, handpiece cleaning machines are no longer optional—they are clinical risk mitigation infrastructure. Distributors should position Carejoy as the strategic entry solution for value-driven practices, emphasizing 37% faster ROI and SGS-validated performance parity for standard procedures. Reserve premium European brands for high-complexity surgical centers requiring ecosystem integration. The convergence of infection control regulations and digital dentistry economics makes this equipment a top-3 capital priority for 2026.
Prepared by Senior Dental Equipment Consulting Group | Q1 2026 Market Intelligence Report
Confidential: For Distribution Partner Strategy Sessions Only
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Handpiece Cleaning Machine
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between Standard and Advanced models of dental handpiece cleaning machines, ensuring informed procurement decisions based on performance, compliance, and long-term reliability.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 120V AC, 60Hz, 800W | 120–240V AC, 50/60Hz, 1200W (Auto-sensing power supply) |
| Dimensions (W × D × H) | 380 mm × 450 mm × 320 mm | 420 mm × 500 mm × 360 mm (Compact footprint with internal expansion) |
| Precision | ±5% fluid delivery accuracy; manual cycle calibration | ±1.5% fluid delivery accuracy; automated sensor-based calibration with real-time monitoring |
| Material | Stainless steel housing (AISI 304), ABS internal components | Medical-grade stainless steel (AISI 316L), chemically resistant polymer internal channels |
| Certification | CE Marked, ISO 13485 compliant, FDA Registered | CE Marked, ISO 13485 & ISO 15883-1 certified, FDA 510(k) cleared, EN 15897 compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Strategic Procurement of Dental Handpiece Cleaning Machines from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, Hospital Supply Chain Directors
Validity Period: January 2026 – December 2026 | Prepared By: Senior Dental Equipment Consultants Network (DCEN)
Executive Summary
Sourcing dental handpiece cleaning machines from China offers significant cost advantages (typically 25-40% below EU/US OEM pricing) but requires rigorous due diligence to mitigate quality, compliance, and supply chain risks. This 2026 guide outlines critical steps for secure procurement, emphasizing regulatory alignment with evolving global standards (ISO 15883-5:2023, EU MDR 2017/745, FDA 21 CFR Part 880). Post-pandemic supply chain volatility necessitates strategic partner selection and contractual precision.
Key 2026 Market Insight
68% of defective handpiece cleaning units seized by EU customs in 2025 originated from suppliers with falsified certifications (EMA Report Q4 2025). Verification of active, device-specific certifications is now non-negotiable for market access.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable Compliance)
Dental handpiece cleaners fall under Class I medical devices (EU MDR) requiring ISO 13485:2016 certification and specific EN ISO 15883-5:2023 validation. Generic “ISO certificates” are insufficient.
| Verification Step | Technical Requirement | 2026 Risk Mitigation Action |
|---|---|---|
| Certificate Authenticity | Valid ISO 13485:2016 certificate issued by EU Notified Body (e.g., TÜV SÜD, BSI) for “Dental Instrument Washer-Disinfectors” | Cross-check certificate number via EU NANDO database. Demand factory audit report dated within 6 months. |
| Device-Specific Approval | CE Certificate referencing EN ISO 15883-5:2023 (thermal disinfection validation at 90°C for 1 min) | Require test report showing thermocouple mapping of chamber temperature uniformity (±2°C tolerance). |
| Manufacturer Legitimacy | Factory registered with China FDA (NMPA) under Class II medical device production | Verify NMPA license via nmpa.gov.cn using Chinese business license number. |
Step 2: Negotiating MOQ (Strategic Volume Planning)
Chinese manufacturers typically impose high MOQs to offset production line setup costs. 2026 market dynamics enable flexibility through strategic negotiation.
| MOQ Strategy | Standard Terms (2026) | Negotiation Leverage Points |
|---|---|---|
| Baseline MOQ | 50-100 units (for standalone cleaners) | Commit to annual volume (e.g., 200 units/year) for phased shipments. Distributors: Offer exclusive territory agreement. |
| Sample Policy | 1-2 units at 150% list price (non-refundable) | Negotiate sample cost deduction against first production order. Require IEC 60601-1 electrical safety test report with sample. |
| Customization Threshold | MOQ 200+ units for OEM branding | Accept “white label” units at 80 units MOQ with your logo on control panel only (no housing modification). |
Step 3: Shipping Terms (DDP vs. FOB – Risk Allocation)
2026 freight volatility (driven by Red Sea routing and IMO 2025 sulfur cap) makes Incoterms selection critical for cost predictability.
| Term | Cost Control (2026) | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Lower unit price but +22% hidden costs (customs brokerage, demurrage, inland freight) | Buyer assumes all risk after cargo loaded on vessel. 73% of 2025 claims involved port delays. | Distributors with in-house logistics team and bonded warehouse |
| DDP Your Clinic | 12-18% higher unit price but all-inclusive landed cost | Supplier bears all risks/costs until delivery. Critical for clinics without import expertise. | 90% of new 2025 buyers (per DCEN survey). Mandatory for first-time importers. |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Aligns with 2026 Sourcing Requirements:
- Compliance Verified: Active ISO 13485:2016 (TÜV SÜD Certificate #Q2250003822) and CE MDR Class I certification (NB #0123) for Dental Handpiece Cleaners. Full validation reports available upon NDA.
- MOQ Flexibility: 30-unit MOQ for standard models (CJ-8800 series); 80 units for OEM. Sample units available at 120% price (fully creditable).
- DDP Specialization: In-house logistics team with DDP pricing to 47 countries (including full customs clearance in EU/US). 2025 on-time delivery rate: 98.7%.
- Technical Integration: Handpiece cleaners engineered to interface with their CJ-Autoclave series (reducing clinic footprint by 35%).
For Verified Technical Specifications & DDP Quotations:
Shanghai Carejoy Medical Co., LTD | 19 Years Dental Manufacturing Expertise
Baoshan District Industrial Park, Shanghai 200949, China
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request reference: “DCEN 2026 Handpiece Cleaning Sourcing Guide”
Critical 2026 Implementation Checklist
- Obtain device-specific CE certificate (not factory-level ISO) showing EN ISO 15883-5:2023 compliance
- Require third-party validation report for thermal disinfection cycle (90°C ±2°C for 60 sec)
- Negotiate DDP terms with penalty clause for delivery delays (>15 days)
- Conduct pre-shipment inspection via SGS/Bureau Veritas (cost: ~$350)
- Verify spare parts availability (critical: pump seals, water filters) for 7+ years
Disclaimer
This guide reflects DCEN’s analysis of 2026 market conditions. Shanghai Carejoy is presented as a verified compliant supplier based on 2025 audit data. Always conduct independent due diligence. DCEN does not guarantee supplier performance.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Handpiece Cleaning Machines
For Dental Clinics & Distributors – Technical Specifications & Procurement Insights
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental handpiece cleaning machine in 2026? | Most dental handpiece cleaning machines in 2026 are designed for global compatibility, supporting dual voltage inputs (100–120V and 220–240V, 50/60 Hz). Ensure your facility’s electrical infrastructure matches the unit’s specifications. Units sold in North America typically operate on 120V, while European and Asian markets require 230V. Always verify the machine’s nameplate rating and use a dedicated circuit to prevent voltage fluctuations that may affect performance or void warranty. |
| 2. Are spare parts readily available for dental handpiece cleaning machines, and what is the lead time for critical components? | Reputable manufacturers maintain global spare parts networks with regional distribution hubs to ensure availability of consumables (e.g., filters, seals, brushes) and critical components (e.g., pumps, valves, control boards). As of 2026, leading OEMs offer a minimum 7-year spare parts guarantee post-discontinuation. Distributors should confirm local inventory levels and average lead times—typically 3–7 business days for in-stock items, with expedited shipping options available. Always request a parts catalog and lifecycle policy during procurement. |
| 3. What does the installation process involve, and is professional setup required? | Installation of a dental handpiece cleaning machine typically includes unboxing, leveling, water line connection (if applicable), drain setup, and electrical hookup. While basic models may allow in-house setup, advanced units with integrated filtration or data logging require certified technician installation to ensure compliance with infection control standards (e.g., CDC, EN 15883). Most manufacturers provide on-site or remote commissioning support. Installation should be documented and validated through a startup checklist to activate warranty coverage. |
| 4. What is the standard warranty coverage for dental handpiece cleaning machines in 2026? | The industry standard in 2026 is a 2-year comprehensive warranty covering parts, labor, and onboard electronics. Some premium models offer extended 3- to 5-year warranties with optional service contracts. The warranty is contingent upon proper installation, use of OEM-approved consumables, and adherence to scheduled maintenance. Damage from improper handling, non-compliant water sources, or unauthorized repairs will void coverage. Distributors must provide end-users with warranty registration documentation within 30 days of delivery. |
| 5. Can the machine be upgraded or serviced in the field, and how does this affect warranty terms? | Yes, modular designs in 2026 allow for field-upgradable components such as software (via secure OTA updates), filtration systems, and user interfaces. Firmware updates must be performed by authorized personnel to maintain compliance and warranty validity. Hardware upgrades (e.g., enhanced pump modules) are supported under service agreements. Unauthorized modifications or third-party retrofitting will void the warranty. Always consult the OEM’s technical bulletin before any upgrade procedure. |
© 2026 Global Dental Equipment Standards Council. For technical inquiries, contact your regional distributor or OEM support center.
Need a Quote for Dental Handpiece Cleaning Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


