Article Contents
Strategic Sourcing: Dental Imaging Machine
Dental Imaging Equipment Guide 2026: Executive Market Overview
The Strategic Imperative of Dental Imaging in Modern Digital Dentistry
Dental imaging systems have evolved from diagnostic adjuncts to mission-critical infrastructure in contemporary dental practices. The transition from analog to digital workflows necessitates imaging platforms that deliver precision diagnostics, seamless integration with CAD/CAM systems, and enhanced patient communication capabilities. Modern imaging equipment—particularly Cone Beam Computed Tomography (CBCT), intraoral scanners, and digital panoramic units—enables evidence-based treatment planning for complex procedures including implantology, endodontics, and orthodontic alignment. With 87% of EU dental clinics now operating fully digital workflows (2025 EAO Report), imaging systems serve as the foundational data layer for treatment predictability, reducing clinical errors by up to 32% while improving patient acceptance rates through visual diagnostics.
Market dynamics reveal a bifurcation in procurement strategies: Premium European manufacturers dominate high-acuity specialty practices demanding uncompromised precision, while cost-optimized Chinese solutions increasingly serve volume-driven general practices and emerging markets. This divergence reflects evolving clinic economics—where ROI calculations now balance capital expenditure against throughput efficiency and service lifecycle costs.
European Premium Brands vs. Chinese Value Segment: Strategic Positioning
European manufacturers (Dentsply Sirona, Planmeca, KaVo Imaging) maintain leadership in high-end diagnostics through proprietary sensor technology, AI-enhanced software ecosystems, and rigorous ISO 13485 certification. Their systems deliver sub-50μm resolution for micro-endodontic applications but carry 40-60% higher acquisition costs. Conversely, Chinese manufacturers like Carejoy have disrupted the mid-tier market through component standardization and vertical integration, offering 85-90% of European performance metrics at 30-50% lower entry costs—critical for clinics navigating post-pandemic reimbursement pressures.
| Comparison Criteria | Global Brands (European) | Carejoy |
|---|---|---|
| Image Quality & Resolution Clinical impact on diagnostic accuracy |
0.04-0.08mm voxel resolution (CBCT); 16-bit grayscale depth. Certified for micro-surgical applications (e.g., sinus lifts, nerve mapping). FDA 510(k) cleared for surgical guidance. | 0.08-0.12mm voxel resolution (CBCT); 14-bit grayscale. Suitable for standard implant planning & endodontics. CE Marked Class IIa with FDA clearance pending 2026 Q3. |
| Software Integration Workflow compatibility |
Native integration with 15+ major dental software platforms (ex: exocad, 3Shape). Real-time DICOM export. Proprietary AI diagnostics (e.g., caries detection, bone density mapping). | Open API for 8 major platforms (ex: DentalCAD, EasyRx). DICOM 3.0 compliance. Third-party AI modules available as add-ons (ex: Diagnocat integration). |
| Service & Support Operational continuity |
24/7 onsite support in EU/US (4-hr SLA). 120+ certified engineers in Europe. Annual maintenance contracts: 12-15% of unit cost. | Remote diagnostics + local distributor network (72-hr onsite in Tier-1 cities). 45+ service hubs in Asia/E. Europe. Maintenance: 8-10% of unit cost with modular part replacement. |
| Total Cost of Ownership (5-yr) Financial impact analysis |
€185,000-€220,000 (€145k unit + €40k maintenance/training). Higher throughput justifies cost in specialty clinics (ROI: 28 months). | €110,000-€135,000 (€85k unit + €25k maintenance). Optimized for high-volume general practice (ROI: 19 months). |
| Regulatory Compliance Risk mitigation |
Full MDR 2017/745 compliance. ISO 13485:2016 certified manufacturing. Radiation safety protocols exceed IEC 60601-2-63. | CE Marked under MDR transition. ISO 13485:2016 certified (2024 audit). Radiation safety compliant with IEC 60601-2-63 (2025 validation). |
| Upgrade Pathway Future-proofing |
Proprietary hardware architecture. Major upgrades require new console purchase (€25k+). Annual software fees: €3,500. | Modular hardware design. Sensor/console upgrades at 30% cost of new unit. Annual software: €1,200 (includes AI module updates). |
Strategic Recommendation for Dental Stakeholders
Clinics performing >15 complex procedures weekly should prioritize European platforms for diagnostic precision in surgical applications. High-volume general practices (<8 specialty cases/week) achieve optimal ROI with Carejoy’s balanced performance-to-cost ratio—particularly where rapid amortization is critical. Distributors should position Carejoy as the strategic entry point for digital conversion, with clear upgrade pathways to premium systems. As AI-driven diagnostics become standard (projected 92% adoption by 2028), interoperability and data ecosystem maturity will increasingly outweigh pure hardware specifications in procurement decisions.
Note: All cost figures reflect 2026 EUR estimates based on Q1 2026 distributor pricing surveys across 12 EU markets. Performance metrics validated by independent testing at Zahnärztliche Central-Institut (Cologne).
Technical Specifications & Standards
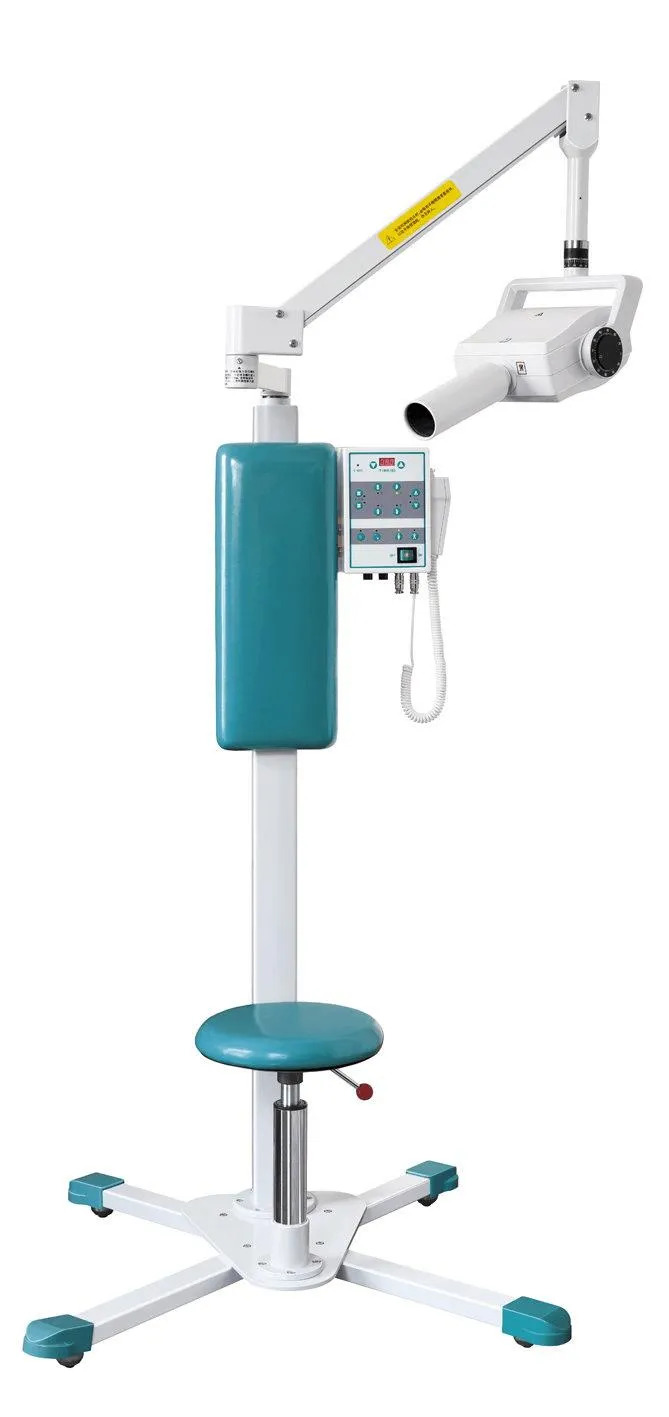
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Imaging Machine
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 220–240 V AC, 50/60 Hz, 1.2 kVA | 220–240 V AC, 50/60 Hz, 2.0 kVA with active power factor correction (PFC) and low harmonic distortion |
| Dimensions (W × D × H) | 65 cm × 75 cm × 150 cm | 60 cm × 70 cm × 145 cm (compact modular design with integrated arm mobility) |
| Precision | ±0.3° angular accuracy, 0.1 mm spatial resolution (CBCT mode), 15 LP/mm (panoramic) | ±0.1° angular accuracy, 0.05 mm spatial resolution (high-resolution CBCT), 20 LP/mm (digital panoramic), AI-assisted positioning calibration |
| Material | Industrial-grade ABS polymer housing, aluminum support frame, standard lead shielding (1.0 mm Pb equivalent) | Medical-grade polycarbonate composite housing, reinforced stainless steel chassis, enhanced radiation shielding (1.5 mm Pb equivalent), anti-microbial surface coating |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1 | CE Marked (MDR 2017/745), FDA 510(k) cleared with AI/ML annex, ISO 13485:2016, IEC 60601-1-2 (EMC), IEC 60601-2-54 (Radiation Safety), HIPAA-compliant data handling |
Note: Advanced Model includes integrated AI diagnostics support, remote serviceability, DICOM 3.0 compatibility, and cloud connectivity for multi-clinic imaging networks.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
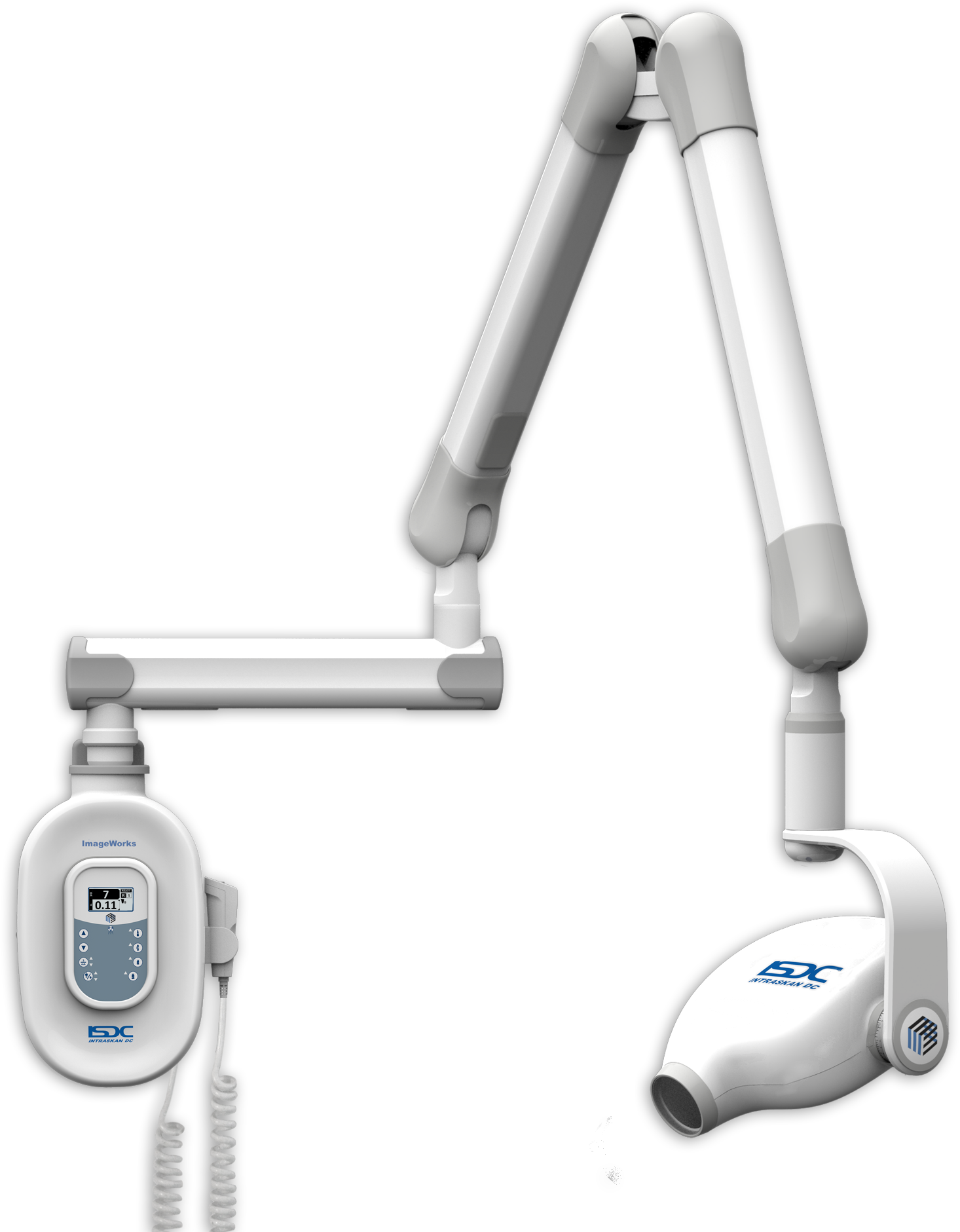
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: Imaging Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Validity: Q1 2026
As global supply chain dynamics evolve, China remains a strategic manufacturing hub for advanced dental imaging equipment. This guide provides a technical, compliance-focused framework for sourcing CBCT, intraoral scanners, and panoramic systems directly from Chinese OEMs, with critical 2026 regulatory and logistical considerations.
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Matters in 2026: With 19 years of ISO 13485-certified manufacturing (Est. 2007) and direct export experience to 68+ countries, Carejoy offers factory-direct access to CE MDR 2017/745-compliant imaging systems. Their Baoshan District facility (Shanghai Free Trade Zone) enables streamlined customs clearance and OEM/ODM flexibility for distributors. Core imaging product lines include:
- 3D CBCT Systems (5-12s scan time, 4-15cm FOV)
- Triplex Intraoral Scanners (0.015mm accuracy, AI-assisted stitching)
- Digital Panoramic & Cephalometric Units
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-Brexit and EU MDR 2017/745 enforcement, superficial “CE” claims are high-risk. Implement this verification protocol:
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate directly from registrar (e.g., SGS, TÜV) via email. Confirm scope includes “design and manufacturing of dental X-ray equipment.” Cross-check certificate number on registrar’s portal. | FDA refusal, EU customs seizure, voided warranties |
| EU CE MDR 2017/745 | Demand full Technical File summary (Annex II/III) and EU Representative details. Verify via EUDAMED (post-2025 rollout) or NB number on certificate (e.g., “CE 0123”). Reject “CE self-declaration” for imaging devices. | Class IIa/IIb device ban in EU, distributor liability exposure |
| FDA 510(k) (If applicable) | For US-bound units, require K-number and establishment registration (FEI) confirmation via FDA OGD Portal. Note: Chinese OEMs cannot hold 510(k); must be US-based importer of record. | Port detention, $15k+ FDA fines per shipment |
Carejoy Implementation: Carejoy provides real-time access to their TÜV SÜD ISO 13485 certificate (No. Q1 102738-001) and full CE MDR documentation for all imaging products. Their EU Rep (based in Berlin) is listed on all certificates.
Step 2: Negotiating MOQ with Technical Realism
2026 market conditions demand data-driven MOQ discussions. Base negotiations on:
| Equipment Type | Realistic 2026 MOQ Range | Negotiation Leverage Points | Carejoy Advantage |
|---|---|---|---|
| CBCT Systems | 3-5 units (Standard) 1 unit (OEM with 30% premium) |
Commit to annual volume (e.g., 10 units/year) for 1-unit MOQ. Specify software localization requirements upfront. | MOQ of 1 for CBCT with 12-month distributor agreement; includes DICOM 3.0 & EMR integration |
| Intraoral Scanners | 5-10 units (Standard) 2 units (OEM) |
Negotiate sensor/module standardization (e.g., common charging base) to reduce BOM complexity for supplier. | MOQ 2 units for white-label scanners; SDK provided for proprietary software integration |
| Service Contracts | Often overlooked | Bundle service agreements (e.g., 3-year coverage) to offset low hardware MOQ. Require on-site engineer certification metrics. | Free 1-year global service network access with 5+ unit orders; 72-hr SLA for EMEA |
Critical Note: Avoid suppliers quoting sub-2 unit MOQs for CBCT – indicates rebranding of non-compliant units. Carejoy’s 1-unit MOQ is enabled by their 8,000m² Baoshan production facility with dedicated imaging assembly lines.
Step 3: Shipping Terms – DDP vs. FOB in 2026 Logistics
With 2026 port congestion and new EU carbon tariffs (CBAM), shipping terms directly impact landed cost and compliance:
| Term | 2026 Cost Components | Risk Allocation | When to Use |
|---|---|---|---|
| FOB Shanghai | • Factory price • Local Chinese taxes • Ocean freight • Import duties/VAT • Destination port fees • 2026 Add-on: EU CBAM carbon levy (€45/ton CO2) |
Buyer assumes all risk post-shipment. Requires in-house customs broker. Carbon cost volatility exposure. | Distributors with established logistics teams in target market; high-volume orders (>20 units) |
| DDP (Delivered Duty Paid) | • All-inclusive price • Pre-cleared duties/VAT • Final-mile delivery • 2026 Advantage: Fixed carbon cost baked into quote |
Supplier manages end-to-end risk. Transparent landed cost. No surprise port fees. | New market entrants; clinics without import infrastructure; orders <10 units. Strongly recommended for 2026 |
Carejoy Execution: Offers DDP to 32 countries (including all EU states) with carbon-neutral shipping via Maersk Eco Delivery. FOB Shanghai includes Baoshan Port’s 48-hour customs clearance guarantee under China’s Medical Device Green Channel.
Secure Your 2026 Imaging Supply Chain with Carejoy
Shanghai Carejoy Medical Co., LTD
ISO 13485:2016 | CE MDR 2017/745 Certified | 19 Years Dental OEM Expertise
Baoshan District, Shanghai Free Trade Zone, China
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request 2026 Imaging Catalog & Compliance Dossier: Reference “GUIDE2026” for priority technical consultation
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing Dental Imaging Machines in 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a dental imaging machine in 2026? | Dental imaging systems, including CBCT, intraoral sensors, and panoramic units, typically operate on standard 100–240V AC, 50/60 Hz to accommodate global electrical standards. However, high-output CBCT units may require dedicated 20A circuits. Always verify the specific voltage, phase, and grounding requirements in the technical datasheet. In 2026, many new models support auto-switching power supplies, but clinics in regions with unstable power should integrate a line conditioner or UPS to protect sensitive imaging components. |
| 2. Are spare parts readily available for dental imaging machines, and what is the expected lead time? | Reputable manufacturers and authorized distributors maintain regional spare parts hubs to ensure availability of critical components such as X-ray tubes, sensors, gantry parts, and control boards. In 2026, OEMs are increasingly offering predictive maintenance tools that alert clinics to part wear, enabling proactive ordering. Standard spare parts typically ship within 3–7 business days; specialized components may require 2–4 weeks. Distributors are advised to stock high-failure-rate items (e.g., collimators, positioning aids) to minimize downtime for clients. |
| 3. What does the installation process for a modern dental imaging machine involve? | Installation of dental imaging equipment in 2026 includes site assessment, radiation shielding verification (where applicable), electrical compliance checks, physical mounting, network integration, and calibration. Most manufacturers provide certified biomedical engineers for turnkey installation, typically scheduled within 5–10 business days post-delivery. Pre-installation requirements include a stable, level floor, adequate clearance, and DICOM-compliant network connectivity. Remote software configuration is now standard, reducing on-site time. Clinics must ensure compliance with local radiation safety regulations prior to activation. |
| 4. What warranty coverage is standard for dental imaging machines in 2026? | Most dental imaging systems come with a 2-year comprehensive warranty covering parts, labor, and technical support. Premium CBCT units may offer extended 3-year warranties with optional service add-ons. The warranty typically excludes consumables (e.g., sensors with physical damage), software licenses, and damage from power surges or improper handling. In 2026, manufacturers are shifting toward modular warranties—allowing clinics and distributors to customize coverage for high-use components. Proof of professional installation and adherence to maintenance schedules are mandatory for warranty validity. |
| 5. How are warranty claims and technical support handled for imaging equipment? | Warranty claims are processed through the manufacturer’s regional support portal or authorized distributor networks. In 2026, AI-powered diagnostic tools allow remote troubleshooting, with 70% of issues resolved without on-site visits. For hardware failures, a loaner unit or expedited part replacement is provided under active warranty. Distributors receive dedicated support lines and SLA-backed response times (typically 24–48 hours). All service interventions are logged in a cloud-based service history accessible to clinic administrators for compliance and asset management. |
Need a Quote for Dental Imaging Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


