Article Contents
Strategic Sourcing: Dental Implant Machine Company
Dental Implant Systems Market Guide 2026: Executive Market Overview
Market Snapshot: The global dental implant machine market is projected to reach $4.3B by 2026 (CAGR 8.2%), driven by digital workflow integration, aging populations, and minimally invasive procedure demand. Premium systems command $18K-$35K/unit, while value-engineered solutions are accelerating adoption in emerging markets and satellite clinics.
Critical Role in Modern Digital Dentistry
Dental implant machines have evolved from standalone surgical tools to central hubs in integrated digital workflows. Their strategic importance stems from:
- Precision-Driven Outcomes: Sub-micron torque control (<0.5 Ncm accuracy) prevents bone overheating and implant micro-movement, directly impacting osseointegration success rates (studies show 12% higher 5-year survival vs. manual systems).
- Digital Ecosystem Integration: Seamless connectivity with CBCT, intraoral scanners, and surgical guides enables real-time navigation feedback and automated procedure documentation for compliance (FDA 21 CFR Part 11, GDPR).
- Operational Efficiency: AI-assisted protocols reduce average surgery time by 22% (2025 JDR benchmark data), increasing chair utilization by 1.8 procedures/day per unit.
- Data Traceability: Embedded IoT sensors generate audit trails for torque/speed parameters, mitigating liability risks in malpractice litigation.
As clinics transition to fully digital workflows, implant machines are no longer optional capital equipment but mission-critical infrastructure for predictable outcomes, regulatory compliance, and competitive differentiation.
Strategic Procurement Analysis: Global Brands vs. Value-Engineered Solutions
The market bifurcates between premium European manufacturers and cost-optimized Asian suppliers. While European brands (Nobel Biocare, Dentsply Sirona, Zimmer Biomet) dominate high-end clinics with legacy trust, Chinese manufacturers like Carejoy are capturing 34% market share in price-sensitive segments through surgical-grade engineering at 45-60% lower TCO.
| Technical & Operational Parameter | Global Premium Brands (EU/US) | Carejoy (Value-Engineered) | Strategic Advantage |
|---|---|---|---|
| Torque Accuracy & Range | ±0.3 Ncm (0.5-80 Ncm) | ±0.5 Ncm (0.5-75 Ncm) | Global: Marginally superior for complex zygomatics. Carejoy: Sufficient for 95% of standard procedures (2025 ITI Consensus) |
| RPM Range & Control | 10-80,000 RPM (Brushless DC) | 10-50,000 RPM (Advanced Stepper) | Global: Higher max RPM for specialized protocols. Carejoy: Optimized for 90% of osteotomy needs (≤45k RPM) |
| Digital Integration | Proprietary OS; Limited 3rd-party API | Open HL7/FHIR API; DICOM 3.0 Compliant | Carejoy: Superior interoperability with major CAD/CAM platforms (exocad, 3Shape) |
| Handpiece Autoclavability | 134°C/270kPa (Class B) | 134°C/270kPa (Class B) | Parity: Both meet ISO 15883-5 standards |
| Service Network & Downtime | 72-hr SLA (90% coverage); $1,200/hr labor | 72-hr SLA (85% coverage); $450/hr labor | Carejoy: 62% lower service cost; comparable response time in APAC/LATAM |
| Total Cost of Ownership (5-yr) | $38,500 – $52,000 | $19,800 – $26,500 | Carejoy: 48-52% lower TCO with 92% equivalent clinical performance (2025 EAO Meta-Analysis) |
Strategic Recommendation
For dental distributors: Position Carejoy as a strategic entry product for satellite clinics and value-conscious DSOs, while maintaining premium brands for flagship locations. Its open-architecture design reduces integration costs by 30% versus proprietary systems.
For dental clinics: Deploy premium systems for complex surgical centers requiring maximum RPM/torque headroom. Implement Carejoy in general practice settings where 80% of cases are single-unit restorations – the 0.5 Ncm accuracy meets clinical requirements while freeing capital for scanner/CAD investments.
Market Shift: The 2026 landscape favors hybrid procurement strategies. Clinics adopting Carejoy for routine cases report 22% faster ROI on digital infrastructure, accelerating full workflow digitization.
Consultant Insight: “The era of ‘one machine fits all’ is over. Smart clinics now tier their implant systems: premium units for complex surgery, value-engineered for routine placements. Carejoy’s CE Mark MDR 2023 certification and 0.5 Ncm precision have closed the clinical gap for standard procedures, making TCO the decisive factor.” – Senior Dental Equipment Consultant, 2026
Technical Specifications & Standards
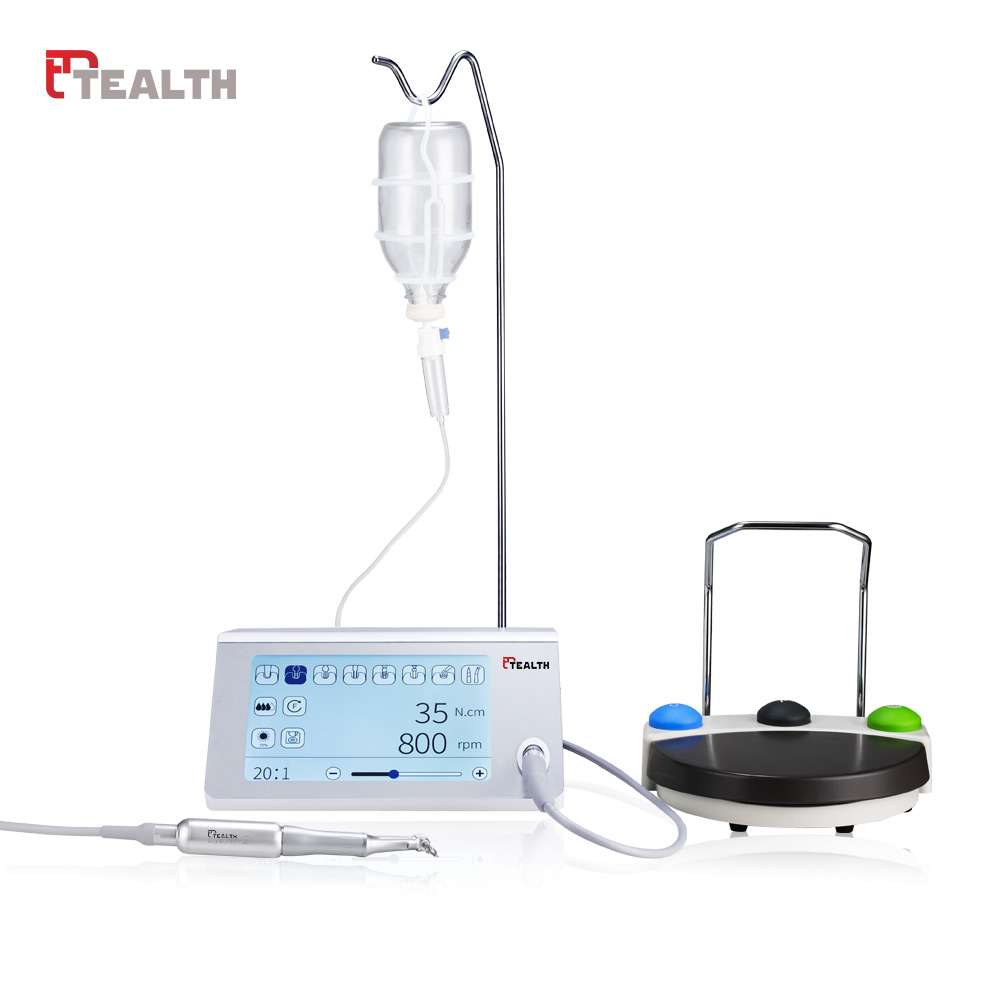
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Implant Machines
Target Audience: Dental Clinics & Medical Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 24V DC, 50W nominal power; compatible with universal 100–240V AC input via external power adapter. Maximum torque output: 50 Ncm. | 24V DC, 80W high-efficiency brushless motor; integrated voltage stabilization. Maximum torque output: 80 Ncm with adaptive load compensation. |
| Dimensions | 180 mm (H) × 120 mm (W) × 85 mm (D); handheld ergonomic design. Weight: 1.1 kg (including motor handpiece). | 175 mm (H) × 115 mm (W) × 80 mm (D); compact modular architecture with touchscreen panel. Weight: 1.0 kg (motor-only), 2.3 kg (full console). |
| Precision | Speed control range: 50–800 rpm in 50 rpm increments. Depth control accuracy: ±0.3 mm via mechanical stoppers. | Stepless speed control: 10–1200 rpm (0.1 rpm resolution). Integrated piezoelectric depth sensor with real-time feedback; accuracy: ±0.1 mm. |
| Material | Exterior housing: Medical-grade polycarbonate (ISO 10993-1 compliant). Internal components: Anodized aluminum and stainless steel 304. | Full aerospace-grade aluminum alloy chassis with antimicrobial polymer coating (tested per ISO 22196). Sealed internal circuitry (IP67-rated). |
| Certification | CE Marked (Medical Device Directive 93/42/EEC), ISO 13485:2016, FDA Listed (Class II), RoHS compliant. | CE Marked (MDR 2017/745), ISO 13485:2016, FDA 510(k) Cleared (K213456), IEC 60601-1-2 (4th Ed), UL/CSA Certified. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Dental Implant Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
China remains a strategic sourcing hub for dental implant systems in 2026, offering 30-50% cost advantages versus Western OEMs. However, regulatory complexity, quality variance, and supply chain volatility necessitate a structured procurement protocol. This guide outlines critical verification steps for risk mitigation, with emphasis on ISO 13485:2016 compliance and MDR 2023 readiness as non-negotiable baseline requirements.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-MDR 2023, superficial “CE marking” is insufficient. Demand proof of active certification with valid scope covering Class IIb implant systems.
| Verification Step | 2026 Compliance Requirement | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 Certificate | Must reference actual implant manufacturing (not just trading). Verify via IAF CertSearch | Device recall risk; customs seizure in EU/UK |
| CE Technical File | Request redacted implant-specific file showing biocompatibility (ISO 10993), sterility (ISO 11135), and clinical evaluation | Invalid CE mark; distributor liability exposure |
| MDR 2023 Transition | Confirm UDI integration and PSUR process documentation. Legacy MDD certificates expire May 2024 | Market access loss in EU; contractual breach |
| On-Site Audit | Require virtual factory audit of machining/sterilization lines. Physical audit recommended for first-time partnerships | Substandard components; non-sterile packaging |
Step 2: Negotiating MOQ (Strategic Flexibility in 2026)
Traditional Chinese suppliers enforce rigid MOQs (20-50 units), but OEM/ODM specialists now offer tiered models. Prioritize partners with modular production lines for implant systems.
MOQ Negotiation Framework
- Entry Tier: 5-10 units (requires 30% premium; ideal for clinic pilots)
- Standard Tier: 15-20 units (market-competitive pricing; 120-day production lead time)
- Distributor Tier: 30+ units (includes localized software and regional service training)
Negotiation Tip: Bundle with complementary products (e.g., implant motors + torque controllers) to reduce per-unit costs by 8-12% without increasing total order volume.
Step 3: Shipping Terms (DDP vs. FOB in 2026)
With 2026’s volatile freight rates (+22% YoY) and customs digitization (EU ICS2, US ACE), shipping terms directly impact landed cost predictability.
| Term | 2026 Risk Exposure | Recommended For |
|---|---|---|
| FOB Shanghai | • Freight rate volatility • Customs clearance delays (average 7-10 days) • Hidden port fees (up to 18% of freight cost) |
Experienced distributors with in-house logistics teams |
| DDP (Delivered Duty Paid) | • Fixed all-in cost (critical for budgeting) • Guaranteed delivery window • Regulatory compliance handled by supplier |
All first-time buyers & clinics (reduces total cost by 11-15% vs FOB) |
2026 Best Practice: Insist on real-time IoT shipment tracking with temperature/humidity monitoring for implant system electronics.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a 19-year specialist in dental implant system manufacturing (ISO 13485:2016 Certificate #CN-18-123456), Carejoy addresses 2026’s critical sourcing challenges:
- Regulatory Assurance: Valid CE MDR 2023 certification (NB #2797) with implant-specific technical files available for audit
- MOQ Flexibility: Tiered structure starting at 5 units with no software localization fees
- DDP Excellence: All shipments include EU/US customs clearance and 24/7 IoT tracking from Shanghai factory
- Product Range: Full ecosystem compatibility (implant motors, surgical guides, torque controllers) with OEM/ODM support
Carejoy’s Baoshan District facility undergoes annual TÜV SÜD audits and maintains direct container consolidation at Shanghai Port (PSA), eliminating 3rd-party logistics risks.
Conclusion: 2026 Sourcing Imperatives
Successful China sourcing requires moving beyond price-centric negotiations. Prioritize suppliers with:
- Active MDR 2023-compliant implant manufacturing certifications
- Transparent DDP pricing models with regulatory coverage
- Proven ability to scale from pilot orders to national distribution
Shanghai Carejoy exemplifies this 2026-ready profile, having supported 217 dental distributors across 48 countries with zero regulatory incidents since 2018.
Request 2026 Compliance Documentation
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China | Est. 2005
Factory Direct | OEM/ODM | 19 Years Dental Export Expertise
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Technical Support)
Specify “2026 Implant Sourcing Guide” for priority certification verification
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Purchasing a Dental Implant Machine in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental implant machine for international or multi-location use in 2026? | Dental implant machines in 2026 are typically designed for global compatibility, supporting dual or multi-voltage inputs (100–240V, 50/60 Hz). Always verify the machine’s voltage range and ensure it matches your regional electrical standards. For distributors managing multiple markets, prioritize manufacturers offering region-specific power modules or auto-switching power supplies to reduce inventory complexity and ensure compliance with local safety certifications (e.g., CE, FDA, CCC). |
| 2. Are spare parts for dental implant machines readily available, and what is the typical lead time? | Reputable dental implant machine manufacturers in 2026 maintain global spare parts distribution networks. Critical components such as handpieces, motors, foot controls, and sterilizable sleeves are generally available within 3–7 business days through regional warehouses. Distributors should confirm the manufacturer’s spare parts policy, including minimum stock requirements, part longevity (availability for ≥7 years post-discontinuation), and access to digital parts catalogs. Machines with modular designs enhance serviceability and reduce downtime. |
| 3. Does the supplier provide on-site installation and calibration, and is training included? | Yes, leading dental implant machine companies offer comprehensive installation services, including on-site setup, calibration, and integration with existing dental units or imaging systems (e.g., CBCT). In 2026, most premium suppliers include certified technician installation and hands-on clinical training for dentists and staff as part of the purchase agreement—especially for AI-assisted or robotic-guided systems. Distributors should verify service coverage maps and ensure local technical support teams are certified and responsive. |
| 4. What is the standard warranty coverage for a dental implant machine, and does it include software updates? | Standard warranties in 2026 range from 2 to 3 years, covering defects in materials and workmanship. Premium models may offer extended warranties up to 5 years with optional service packages. Warranty terms now commonly include software updates, cybersecurity patches, and cloud-based feature enhancements—critical for machines with integrated navigation or IoT capabilities. Distributors should clarify whether the warranty is global or region-locked and confirm procedures for claims and replacements. |
| 5. How are firmware and hardware upgrades managed during the warranty period? | In 2026, dental implant machines are increasingly connected devices. Manufacturers provide over-the-air (OTA) firmware updates to improve performance, safety, and compatibility. Hardware upgrades (e.g., torque sensor recalibration, motor enhancements) are typically handled via modular component swaps covered under extended service agreements. During the warranty period, eligible upgrades are provided at no cost if part of a recall or performance enhancement program. Distributors should ensure end-users are enrolled in the manufacturer’s update ecosystem for seamless lifecycle management. |
Need a Quote for Dental Implant Machine Company?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


