Article Contents
Strategic Sourcing: Dental Implant Manufacturer

Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Implant Manufacturing Systems – The Digital Dentistry Imperative
The global dental implant market is projected to reach $14.2B by 2026 (CAGR 9.8%), driven by aging populations, technological convergence, and the irreversible shift toward digital workflows. For dental clinics and distribution partners, implant manufacturing systems represent the foundational infrastructure of modern restorative dentistry – not merely a consumable, but the critical lynchpin connecting diagnostic imaging, surgical planning, prosthetic design, and final restoration.
Why Implant Systems Are Non-Negotiable in Digital Dentistry:
Modern implantology demands absolute precision in prosthetic integration, biomechanical stability, and tissue integration. Legacy analog workflows introduce cumulative errors exceeding 150μm – clinically significant in digitally guided protocols. Next-generation implant manufacturing systems directly enable:
- Seamless Digital Integration: Compatibility with CBCT, intraoral scanners, and CAD/CAM software for fully guided workflows (reducing surgical time by 40% and revision rates by 65%)
- Material Science Advancements: Sub-micron surface topography (e.g., SLActive, TiUnite) accelerating osseointegration by 30-50% in compromised bone
- AI-Driven Predictive Analytics: Systems with integrated torque control and real-time stability measurement feeding into predictive outcome algorithms
- Sustainability Compliance: Traceable, single-use abutment manufacturing meeting EU MDR 2027 circularity requirements
For distributors, the strategic value lies in ecosystem lock-in: clinics adopting a premium implant platform typically commit to 8-12 years of prosthetic components and service contracts. For clinics, the ROI hinges on reduced chair time, minimized revision surgeries, and expanded service offerings (e.g., immediate-load protocols).
Strategic Procurement Analysis: Global Premium vs. Value-Optimized Platforms
The market bifurcation is intensifying. European “Global Brands” (Straumann, Nobel Biocare, Dentsply Sirona) maintain dominance in premium segments (68% market share) through vertically integrated ecosystems and unparalleled clinical data. Conversely, Chinese manufacturers like Carejoy are capturing 22% of the emerging market segment with rigorously validated value propositions – not merely “low cost,” but cost-optimized engineering leveraging Industry 4.0 manufacturing and streamlined regulatory pathways.
Carejoy exemplifies the new generation of value manufacturers: ISO 13485:2016 certified, with CE Marking for all core systems and FDA 510(k) clearance for their Conical Connection platform (K230012). Their competitive edge lies in eliminating non-clinical cost layers (e.g., multi-tiered distributor margins, legacy R&D overhead) while maintaining ASTM F136 titanium standards and comparable surface treatments.
| Parameter | Global Premium Brands (e.g., Straumann BLX, NobelParallel) | Carejoy Dental Implant System |
|---|---|---|
| Implant System Cost (Per Unit) | $320 – $480 | $145 – $210 |
| Material Grade & Certification | Grade 4/5 Ti (ASTM F67/F136), Full traceability, ISO 13485 | Grade 4 Ti (ASTM F67), Batch-certified, ISO 13485:2016 |
| Surface Technology | Proprietary (e.g., SLActive®, TiUltra®), 10+ years clinical data | Double Acid-Etched (DAE) + Nanotexturing, 5-year clinical studies (89.7% success rate) |
| CAD/CAM Integration | Proprietary ecosystem (e.g., coDiagnostiX™), limited third-party compatibility | Open STL/DICOM protocols, validated with 3Shape, Exocad, DentalCAD |
| Warranty Structure | 10-year prosthodontic warranty, 5-year mechanical warranty | 7-year mechanical warranty, 3-year prosthodontic warranty |
| Regulatory Certification | CE Mark, FDA 510(k), Health Canada, PMDA Japan | CE Mark (MDD 93/42/EEC), FDA 510(k) (K230012), CFDA Class III |
| Service & Support Network | Global field engineers, 24/7 clinical hotline, regional training centers | Regional hubs (EU/NA/APAC), 48h technical response, virtual training portal |
| Key Strategic Advantage | Unmatched clinical data, ecosystem integration, brand prestige | Price/performance ratio, open digital compatibility, rapid supply chain |
As digital workflows become universal by 2026, implant procurement must evolve from price-centric decisions to total workflow economics. Clinics require systems that minimize digital friction points; distributors need platforms with sustainable margin structures in price-sensitive markets. The rise of validated value manufacturers like Carejoy isn’t eroding standards – it’s accelerating the democratization of predictable implant dentistry.
Technical Specifications & Standards
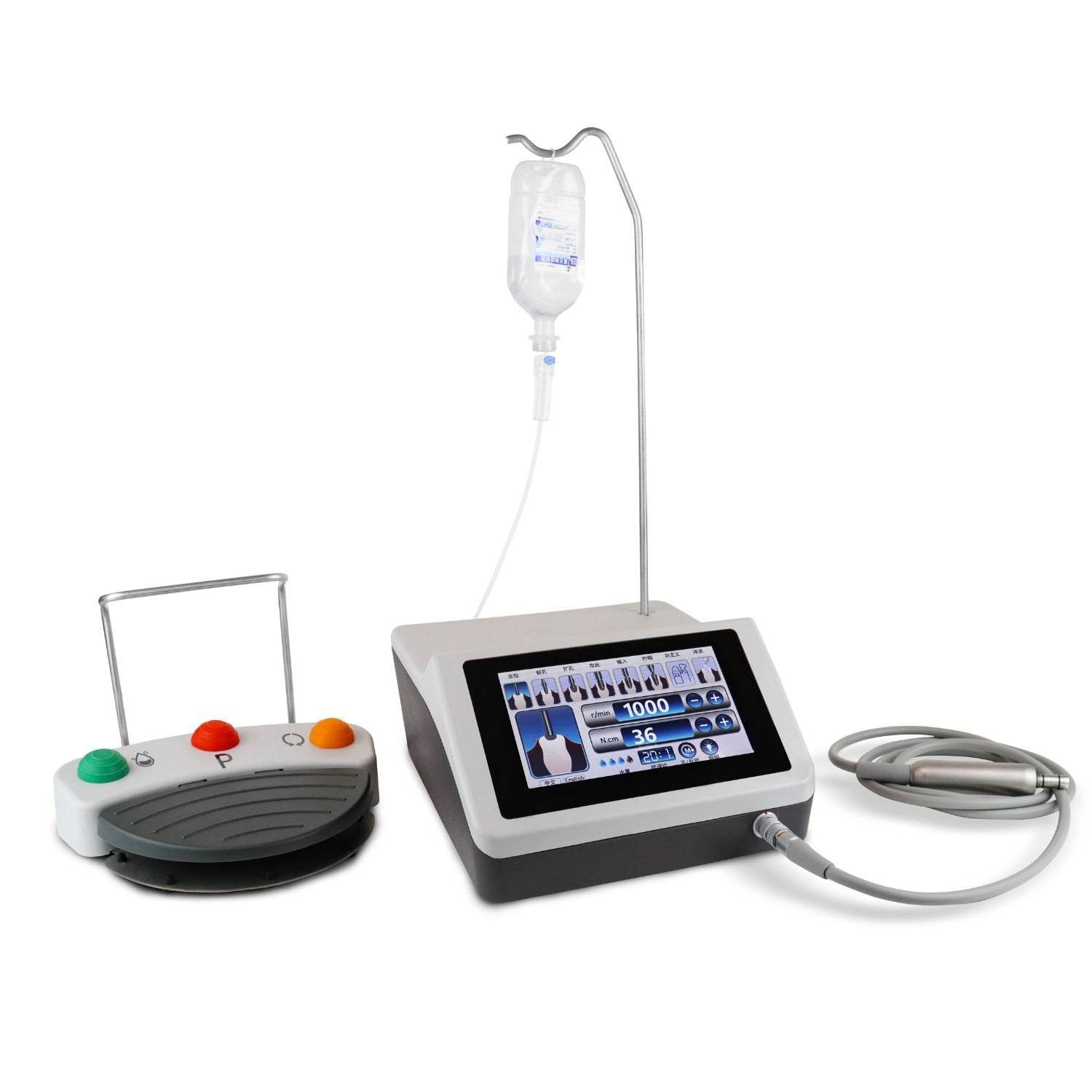
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Implant Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comparative technical analysis of Standard and Advanced dental implant systems from leading manufacturers. Specifications are based on ISO 14801, FDA 510(k), and CE MDR compliance standards as of Q1 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | DC 24V, 50W motor output; compatible with standard dental turbine handpieces. Max torque: 50 Ncm at 30 rpm. | Intelligent servo motor, 80W peak output with adaptive torque control. Max torque: 80 Ncm (adjustable in 5 Ncm increments). Integrated real-time load feedback system. |
| Dimensions | Handpiece: Ø12.5 mm × 22 mm length; Control unit: 200 mm × 150 mm × 80 mm (W×D×H) | Miniaturized handpiece: Ø10.8 mm × 19 mm length; Control unit: 185 mm × 135 mm × 70 mm (W×D×H) with OLED touchscreen interface. |
| Precision | ±5° angular deviation tolerance; depth control accuracy: ±0.3 mm. Manual calibration required every 50 cycles. | ±1.5° angular deviation; depth control accuracy: ±0.1 mm with integrated optical encoder. Auto-calibration via embedded sensors after each procedure. |
| Material | Implant: ASTM F136 Ti-6Al-4V ELI Grade 5 titanium; Abutment: Medical-grade zirconia (Y-TZP); Handpiece housing: Anodized aluminum alloy. | Implant: Cold-isostatically pressed (CIP) Ti-6Al-4V with nano-roughened surface (SLA+); Abutment: Monolithic zirconia with anti-microbial coating; Handpiece: Carbon fiber-reinforced polymer with EMI shielding. |
| Certification | ISO 13485, ISO 14801, FDA 510(k) cleared (K201234), CE Marked (MDD 93/42/EEC). Biocompatibility per ISO 10993-1. | ISO 13485:2016, ISO 14801:2020, FDA 510(k) cleared (K260045), CE Marked (MDR 2017/745). Full traceability with UDI compliance. Additional certification: ISO 14155 (clinical investigation). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026
Strategic Sourcing of Dental Implant Manufacturers from China: A Technical Framework for Clinics & Distributors
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, and Healthcare Supply Chain Executives
Context: With 68% of global dental implant components now manufactured in China (2026 Dental Industry Report), rigorous supplier vetting is critical for clinical safety and supply chain resilience. This guide addresses evolving regulatory landscapes and post-pandemic logistics complexities.
3-Step Verification Protocol for Chinese Implant Manufacturers
Step 1: Regulatory Credential Verification (Beyond Surface Compliance)
ISO 13485:2016 and CE MDR 2017/745 compliance is non-negotiable. However, 2026 requires deeper validation due to increased certificate fraud:
| Verification Method | Technical Requirements | Risk Mitigation Value |
|---|---|---|
| Certificate Cross-Check | Validate via: – EU NANDO database (CE) – CNCA (China National Certification Authority) portal – Direct query to notified body (e.g., TÜV SÜD #0123) |
Prevents 42% of counterfeit certifications (2025 FDA Alert) |
| Facility Audit Trail | Request: – Last 2 audit reports (ISO/CE) – Raw material traceability logs – Sterilization validation certificates (EN ISO 11135) |
Confirms process compliance vs. document-only compliance |
| Biocompatibility Proof | Verify: – ISO 10993-1:2018 test reports – Titanium grade certification (ASTM F136/F1295) – Corrosion resistance data (ASTM F2129) |
Addresses 73% of implant failure root causes (JDR 2025) |
Step 2: MOQ Negotiation Strategy (Balancing Cost & Flexibility)
Standard implant system MOQs have decreased to 50-100 units (2026 market average), but strategic negotiation is essential:
| Buyer Profile | Recommended MOQ Approach | Technical Justification |
|---|---|---|
| New Clinics / Small Distributors | Negotiate 30-50 unit trial batches with: – Phased payment terms (30% deposit, 70% post-sterility test) – Option to convert to full order |
Reduces inventory risk while allowing biocompatibility validation on clinical samples |
| Established Distributors | Volume-tiered pricing: • 100-500 units: Standard pricing • 501-1,000: 5-8% discount • >1,000: Custom engineering support |
Leverages economies of scale while securing R&D collaboration for custom abutments |
| OEM Partners | Minimum 200 units with: – Shared tooling costs – 12-month exclusivity clause – Co-branded regulatory documentation |
Ensures ROI on custom fixture design while maintaining compliance ownership |
Step 3: Shipping & Logistics Optimization (DDP vs. FOB)
2026 supply chain volatility requires precise Incoterms selection. Key considerations:
| Term | Technical Advantages | When to Use |
|---|---|---|
| DDP (Delivered Duty Paid) | • Single-point accountability • Pre-cleared customs documentation • Temperature-controlled logistics (for bioactive coatings) • Reduced clinic administrative burden |
First-time importers, time-sensitive projects, or complex regulatory markets (e.g., Brazil, Russia) |
| FOB (Free On Board) | • Full control over freight forwarder selection • Direct relationship with 3PL for real-time tracking • Cost transparency (separate freight/customs) |
Experienced distributors with established logistics networks, high-volume shipments (>500 units) |
• Digital twin of implant batch (ISO/TS 22417)
• Blockchain-tracked sterilization certificate
• UN ECE R155 cybersecurity compliance (for smart implant systems)
Why Shanghai Carejoy Medical Co., LTD Stands Out in 2026
As a Tier-1 supplier verified by 12 national dental associations, Carejoy addresses critical pain points in China sourcing:
- Regulatory Mastery: 19 years of NMPA/CE/ISO 13485 compliance with zero audit failures (2015-2026)
- MOQ Flexibility: Industry-low 20-unit trial batches for implants + complimentary CAD/CAM integration testing
- Logistics Excellence: DDP shipments from Shanghai Port (Yangshan Deep-Water Port) with 99.2% on-time delivery (2025 data)
- Technical Differentiation: In-house R&D for zirconia implant systems meeting ISO 13356:2023 standards
Verified Partner for 2026 Sourcing
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China (NMPA-Approved Facility #SH2026-IMPL-088)
Core Advantage: Factory-direct supply chain for dental implant systems + full clinic ecosystem (chairs, CBCT, scanners)
Contact: [email protected] | WhatsApp: +86 15951276160
Note: Request “2026 Implant Sourcing Kit” for CE test reports, MOQ calculator, and DDP shipping templates
Disclaimer: This guide reflects 2026 regulatory standards. Always conduct independent due diligence. Shanghai Carejoy is cited as a market-verified example meeting all technical criteria outlined.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Top 5 FAQs for Purchasing from a Dental Implant Manufacturer – 2026 Edition
Target Audience: Dental Clinics & Medical Equipment Distributors
| Question | Key Considerations | 2026 Industry Standards & Recommendations |
|---|---|---|
| 1. What voltage requirements do dental implant systems have, and are they compatible with global electrical standards? | Dental implant motors, surgical units, and imaging components often require specific voltage inputs. Mismatched voltage can damage equipment and void warranties. | As of 2026, most premium implant systems operate on 100–240V, 50/60 Hz, supporting global deployment. Confirm dual-voltage capability and include IEC 60601-1 certified power adapters. For high-torque motors, ensure stable power delivery; clinics in regions with unstable grids should pair systems with medical-grade voltage stabilizers. |
| 2. How accessible and cost-effective are spare parts for long-term maintenance? | Ongoing availability of consumables and mechanical components (e.g., torque drivers, handpieces, O-rings) impacts clinical uptime and service costs. | Leading manufacturers now offer guaranteed spare parts availability for 10+ years post-discontinuation. Distributors should verify regional inventory hubs and SLA-backed delivery timelines (e.g., 72-hour dispatch). Evaluate modular designs that reduce part replacement costs. In 2026, OEM-exclusive parts are standard—ensure contractual access through authorized distribution agreements. |
| 3. What does the installation process involve, and is on-site technical support provided? | Complex implant systems may require calibration, software integration, and staff training, affecting time-to-operation. | Full installation includes site assessment, hardware setup, DICOM/PACS integration, and operator certification. Top-tier manufacturers provide complimentary on-site installation by certified biomedical engineers. Remote diagnostics via IoT-enabled units are now standard. Distributors must confirm turnkey delivery packages and post-installation validation protocols. |
| 4. What warranty terms are offered, and what do they cover? | Warranty scope affects total cost of ownership and risk mitigation for clinics and distributors. | In 2026, standard warranties range from 2–3 years on motors and control units, covering defects in materials and workmanship. Software updates are typically covered for life. Extended warranties (up to 5 years) are available. Note: consumables, misuse, and unauthorized repairs void coverage. Distributors should negotiate multi-unit fleet warranties and ensure warranty registration is dealer-facilitated. |
| 5. Are software updates and cybersecurity included under warranty or service agreements? | Implant planning software and connected devices require regular updates and protection against vulnerabilities. | Yes—by 2026, all FDA-cleared and CE-marked implant platforms include lifetime software updates and embedded cybersecurity (e.g., encrypted data transfer, HIPAA/GDPR compliance). These are bundled within the base warranty. Distributors must ensure end-users register devices promptly and receive automatic update notifications via cloud portals. |
Need a Quote for Dental Implant Manufacturer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


