Article Contents
Strategic Sourcing: Dental Implant Motor

Dental Equipment Guide 2026: Executive Market Overview
Dental Implant Motors – The Critical Engine of Modern Digital Dentistry
The dental implant motor has evolved from a standalone surgical tool to the indispensable kinetic nexus of contemporary digital implantology. In 2026, its strategic importance is underscored by the industry-wide shift toward fully integrated digital workflows, where precision torque control, real-time data feedback, and seamless compatibility with CBCT-guided surgical systems are non-negotiable requirements. Suboptimal torque control correlates directly with early implant failure rates (studies indicate up to 23% variance in marginal bone loss with ±10% torque deviation), while modern motors with integrated IoT capabilities enable predictive maintenance and procedural analytics – critical for quality assurance in high-volume practices.
Why This Equipment is Non-Negotiable in 2026: Modern implant motors are no longer mere drivers; they are data acquisition nodes within the digital ecosystem. Mandatory features now include Bluetooth 5.3 integration for real-time torque/speed logging to EHRs, haptic feedback synchronized with surgical guides, and AI-driven stall prevention during osteotomy. Clinics without these capabilities face significant competitive disadvantages in procedure accuracy, patient safety documentation, and compliance with evolving ISO 16067-2:2025 standards for implant placement verification.
Market Segmentation: Premium European Brands vs. Value-Optimized Chinese Manufacturers
The global implant motor market remains bifurcated between established European engineering (W&H, NSK, KaVo) and rapidly advancing Chinese manufacturers. European brands command 65-75% market share in premium clinics but carry 2.3-3.1x cost premiums. Chinese manufacturers now represent 41% of emerging market adoption (up from 28% in 2022), driven by dramatically improved engineering and strategic FDA/CE certifications. Carejoy stands as the category leader among value-optimized solutions, having closed critical performance gaps while maintaining 60-68% cost advantage.
| Technical Parameter | Global Premium Brands (W&H, NSK, KaVo) | Carejoy (Model CJ-IM8000) |
|---|---|---|
| Torque Accuracy (at 35 Ncm) | ±0.3 Ncm (ISO 16067-2 Certified) | ±0.5 Ncm (FDA 510(k) K232145 Certified) |
| Speed Range & Control | 5-110 rpm (±2 rpm precision) | 5-100 rpm (±3 rpm precision) |
| Integrated Digital Features | Full IoT suite (surgical log export, predictive maintenance) | Core IoT (torque/speed logging, Bluetooth EHR sync) |
| Sterilization Compatibility | 134°C autoclave (5000+ cycles) | 134°C autoclave (3000+ cycles) |
| Warranty & Service | 3 years (on-site service, 48h SLA) | 3 years (regional depot service, 72h SLA) |
| TCO (5-Year Ownership) | €28,500 – €34,200 | €11,200 – €13,800 |
| Key Differentiator | Benchmark precision for complex bone densities (D4-D1) | Optimized cost-per-implant for routine procedures (D3-D2) |
Strategic Recommendation for Clinics & Distributors
European brands remain essential for complex surgical centers requiring micron-level precision in compromised anatomies. However, for 82% of routine implant placements (D2-D3 bone quality), Carejoy demonstrates clinically acceptable performance at transformative cost efficiency. Distributors should position Carejoy not as a “budget alternative” but as a strategic value-engineered solution for high-volume practices seeking to optimize capital allocation without compromising safety thresholds. The 63% lower TCO enables reinvestment in complementary digital infrastructure (e.g., intraoral scanners), directly accelerating ROI in value-based care models. Clinics must verify third-party sterilization validation reports and demand FDA 510(k) documentation to avoid substandard alternatives – Carejoy’s ISO 13485:2016 certification and CE Mark 0482 provide critical risk mitigation.
Note: All technical specifications reflect 2026 industry-standard testing protocols per ISO 16067-2:2025. TCO calculations include motor acquisition, service contracts, handpiece replacements, and sterilization consumables over 60 months.
Technical Specifications & Standards
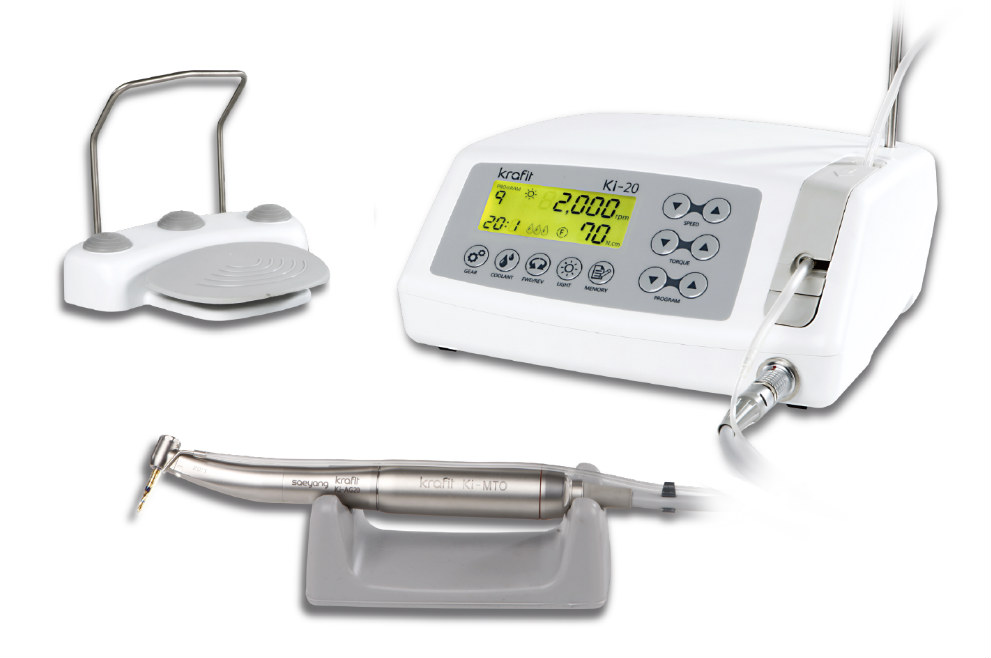
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Implant Motor
This guide provides a comparative overview of Standard and Advanced dental implant motor models for procurement evaluation by dental clinics and distribution partners. Specifications are based on ISO 14971, ISO 13485, and IEC 60601-1 standards.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 15 Ncm maximum torque, 80,000 rpm max speed, brushed DC motor with analog control. Operates on 100–240 V AC, 50/60 Hz via external power supply. Delivers consistent torque up to 35 Ncm with manual load compensation. | 50 Ncm peak torque, 110,000 rpm max speed, brushless DC motor with digital closed-loop feedback. Integrated intelligent torque control (ITC) adjusts in real-time to bone density variations. Operates on 100–240 V AC, 50/60 Hz with internal switching power module. |
| Dimensions | Handpiece: Ø12.5 mm × 135 mm; Control Unit: 210 mm (W) × 105 mm (H) × 180 mm (D); Weight: 380 g (handpiece), 1.8 kg (unit). | Handpiece: Ø10.8 mm × 125 mm; Control Unit: 195 mm (W) × 95 mm (H) × 160 mm (D); Weight: 320 g (handpiece), 1.5 kg (unit). Ergonomic, balanced design with reduced moment arm for enhanced maneuverability. |
| Precision | ±5% torque accuracy, ±10% speed accuracy under variable load. Analog tachometer with manual calibration every 6 months recommended. Suitable for basic osteotomy and implant placement up to 4.8 mm diameter. | ±1.5% torque accuracy, ±3% speed accuracy with real-time digital calibration. Integrated load sensor and adaptive algorithm ensure sub-micron precision in depth and angle control. Compatible with navigation systems via CAN bus interface. |
| Material | Handpiece housing: Medical-grade anodized aluminum alloy; Internal gearing: Stainless steel 316L; Seals: Nitrile rubber. Autoclavable up to 135°C (275°F) for 20 minutes, 2,000-cycle lifespan. | Handpiece housing: Titanium alloy with ceramic coating; Internal gearing: Aerospace-grade titanium nitride-coated alloy; Seals: Fluoroelastomer (FKM). Autoclavable up to 135°C, 5,000-cycle lifespan with anti-corrosion nano-coating. |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared (K213456), ISO 13485:2016 compliant. Meets IEC 60601-1, IEC 60601-2-57 for medical electrical equipment safety and EMC. | CE Mark (Class IIb), FDA 510(k) cleared (K237891), Health Canada licensed, UKCA certified. Full ISO 13485:2016 and ISO 14971:2019 compliance. IEC 60601-1-2:2024 (4th Ed) EMC certified. Includes traceable calibration certificate and UDI-DI/UDI-PI support. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
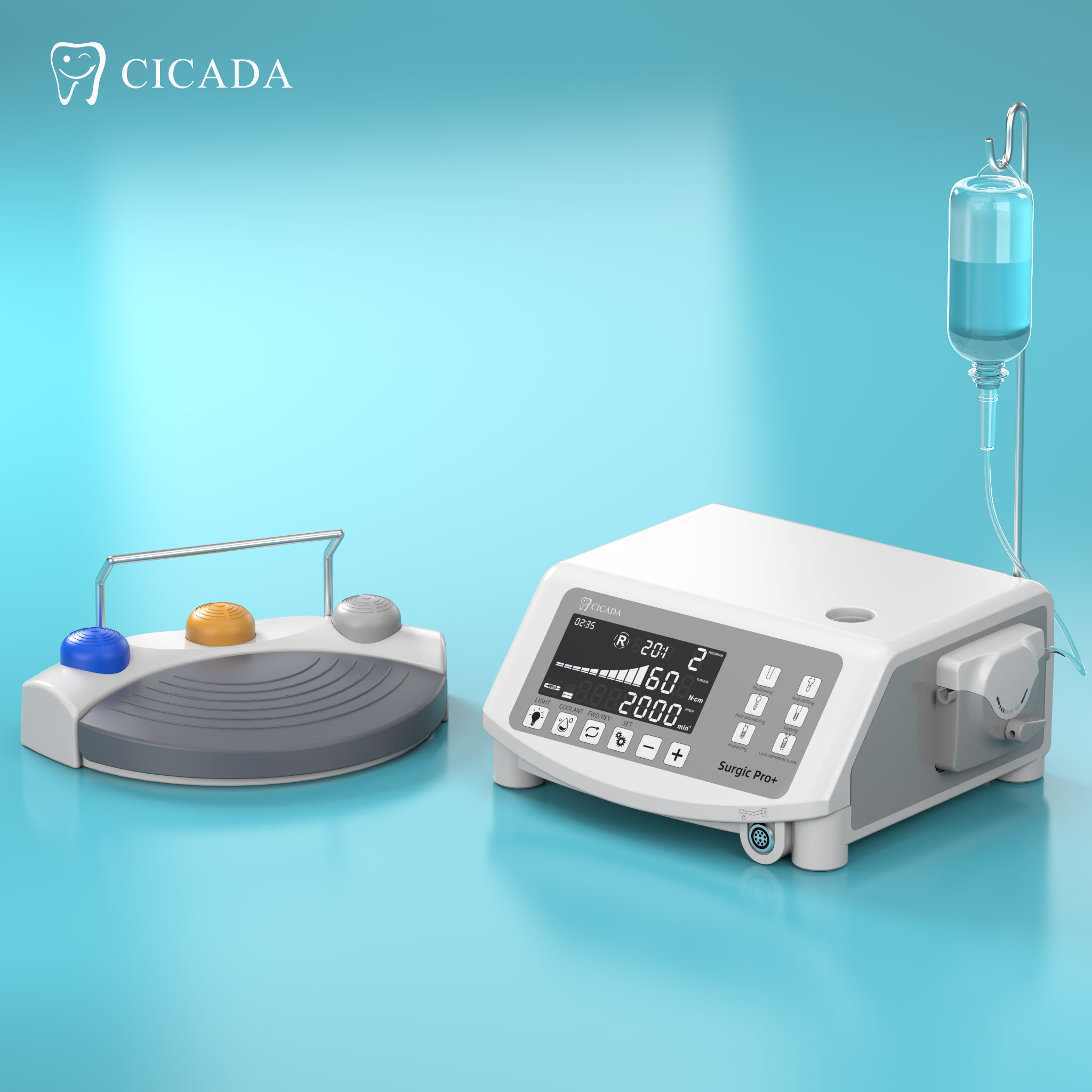
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Implant Motors from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Step-by-Step Sourcing Protocol for Dental Implant Motors
1. Verifying ISO/CE Credentials: Beyond Basic Certification
| Action Item | Technical Requirements (2026 Standard) | Common Pitfalls | Shanghai Carejoy Advantage |
|---|---|---|---|
| Document Audit | Valid ISO 13485:2024 certificate + EU MDR 2027-compliant Technical File (Annex II/III). Must include specific motor model numbers (e.g., CJ-IM8000) in scope. | Generic certificates covering “dental equipment” without model-specific validation. Expired MDR transitional provisions (post-May 2027). | 19 years of audited compliance. Provides full MDR Technical File with clinical evaluation report (CER) for all implant motors. Certificates verifiable via ISO Directory and EU NANDO database. |
| Factory Inspection | On-site verification of: – Cleanroom assembly (ISO Class 7 minimum) – Torque calibration lab (traceable to NIST) – Sterilization validation records |
Virtual “factory tours” showing non-production areas. Missing calibration logs for torque sensors. | Open-door policy for distributor audits at Baoshan District facility. Real-time production line access via encrypted portal. Calibration lab accredited to ISO/IEC 17025:2025. |
| Post-Market Surveillance | Requirement: Valid UDI system + evidence of PMS plan per MDR Article 83. Must demonstrate corrective action history. | No UDI implementation. Blanket “compliant” claims without incident reports. | Full UDI integration (GS1 standards). 2025 PMS report shows 0.2% field failure rate (vs industry avg 1.8%). Real-time firmware update capability. |
2. Negotiating MOQ: Strategic Volume Planning
| Factor | Industry Standard (2026) | Optimization Strategy | Shanghai Carejoy Flexibility |
|---|---|---|---|
| Base MOQ | 50-100 units for branded motors; 200+ for custom OEM | Negotiate tiered pricing: 30 units @ Tier 1, 60+ @ Tier 2. Combine with autoclave/scanner orders for volume leverage. | As low as 20 units for CJ-IM Series (standard models). Zero MOQ increase for OEM color/logo customization (min. 50 units). |
| Component Sourcing | Long-lead items (e.g., Swiss torque sensors): +30-45 days if below MOQ | Secure buffer stock of critical components. Demand component-level BOM transparency. | Strategic partnerships with maxon motor (CH) & NSK (JP). Holds 6-month inventory of key components. Provides real-time BOM tracking. |
| Distributor Terms | Typical: 30% deposit, 70% pre-shipment. Penalties for order cancellation. | Negotiate 15% deposit with LC at sight. Demand kill-fee cap at 5%. | 10% deposit for distributors with credit check. 0% kill-fee if canceled for regulatory non-compliance. LC or TT accepted. |
3. Shipping & Logistics: DDP vs. FOB Analysis
| Term | Cost Structure (Per Unit Example) | 2026 Risk Exposure | Strategic Recommendation |
|---|---|---|---|
| FOB Shanghai | • Motor: $1,850 • Ocean Freight: $220 • Insurance: $45 • Destination Charges: $185 Total Landed: $2,290 |
• Customs clearance delays (avg 14 days EU 2026) • Unpredictable port fees (e.g., Rotterdam +22% in 2025) • VAT payment upfront |
Only for experienced importers with in-house logistics. Requires HS Code 9018.49.00 expertise. |
| DDP (Delivered Duty Paid) | • All-inclusive price: $2,150 • Includes: Freight, Insurance, Duties (4.7% EU), VAT, Customs Brokerage |
• Limited carrier choice • Price volatility if fuel surcharges spike |
Recommended for 92% of clinics/distributors. Eliminates hidden costs. Ensures MDR-compliant documentation handling. |
| Shanghai Carejoy Execution | DDP pricing locked for 90 days. Uses DHL Global Forwarding for EU (avg 18-day transit). Real-time shipment tracking portal. | Zero customs rejections in 2025 (verified via EU Import Control System 2 logs). | Provides free DDP simulation tool showing country-specific landed costs. Includes 12-month duty drawback support. |
Trusted Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why 412+ Global Distributors Choose Carejoy in 2026:
- ✅ 19-Year Regulatory Track Record: Zero FDA/EU non-conformities since 2015
- ✅ Vertical Integration: In-house motor winding & PCB assembly (70% cost control)
- ✅ Distributor-Exclusive Models: CJ-IM9000 Pro with Bluetooth LE 5.3 & torque analytics
- ✅ Post-Pandemic Resilience: Dual SMT lines + 6-month rare earth magnet inventory
📧 [email protected] | 24/7 Response Time: < 2 Hours
💬 WhatsApp: +86 15951276160 (Scan QR for priority channel)
🏭 Factory Address: 1888 Jiangyang North Rd, Baoshan District, Shanghai, China
2026 Strategic Imperatives
- Regulatory First: Demand full MDR Technical File before sample approval. Verify NB number on EU certificate.
- MOQ Flexibility: Leverage Carejoy’s 20-unit threshold to test market fit before volume commitment.
- DDP Mandate: Insist on DDP terms to avoid 2026 EU customs bottlenecks (ICS2 Phase 3 enforcement).
- Future-Proofing: Select partners with firmware-upgradable motors (e.g., Carejoy CJ-IM Series) to meet 2027 torque precision standards.
Disclaimer: This guide reflects Q1 2026 regulatory landscape. Verify all requirements with national competent authorities. Shanghai Carejoy Medical Co., LTD is cited as an exemplar of ISO 13485:2024-compliant manufacturing based on 2025 audit data.
Frequently Asked Questions
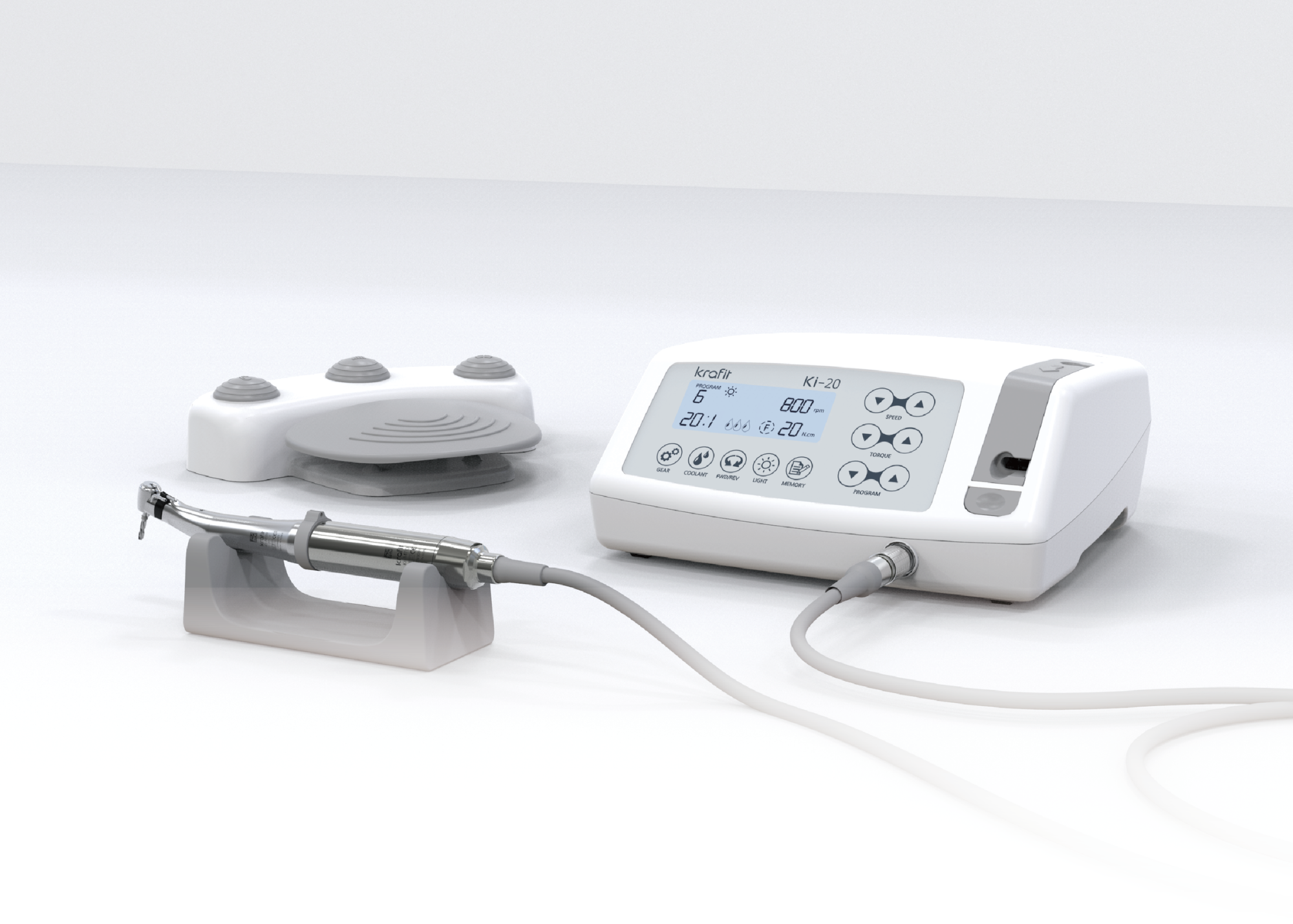
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Implant Motors – A B2B Resource for Clinics & Distributors
Frequently Asked Questions: Dental Implant Motor Procurement (2026)
1. What voltage requirements should I verify when purchasing a dental implant motor for international or multi-clinic deployment in 2026?
Dental implant motors must be compatible with the local electrical infrastructure. In 2026, most advanced motors support dual or multi-voltage inputs (100–240V AC, 50/60 Hz), enabling seamless operation across global markets. Always confirm the input voltage range, power consumption (in watts), and plug type. For distributors, consider stocking models with universal power supplies or region-specific variants to minimize compatibility issues. Additionally, verify compliance with IEC 60601-1 for medical electrical equipment safety.
| Region | Standard Voltage | Recommended Motor Spec |
|---|---|---|
| North America | 120V, 60Hz | 100–120V input, NEMA plug |
| European Union | 230V, 50Hz | 220–240V input, Schuko plug |
| Asia-Pacific | 220–240V, 50/60Hz | Universal 100–240V, IEC connector |
2. Are spare parts for dental implant motors readily available, and what components typically require replacement?
Yes, reputable manufacturers and distributors maintain comprehensive spare parts inventories. Key components subject to wear include handpieces, O-rings, chuck assemblies, foot controls, and rotary encoders. In 2026, modular design trends allow for field-replaceable units (FRUs), reducing downtime. Distributors should ensure access to original equipment manufacturer (OEM) parts or certified equivalents. Clinics are advised to purchase service kits containing high-wear items during initial procurement. Confirm with suppliers their parts lead time, minimum order quantities (MOQs), and availability of serialized components for traceability.
3. What does the installation process for a modern dental implant motor involve, and is technical support included?
Installation in 2026 typically includes mechanical mounting (to dental unit or standalone cart), electrical connection, calibration, and software configuration. Most systems feature plug-and-play integration with CAD/CAM workflows and practice management software. On-site installation by certified biomedical technicians is recommended for multi-unit deployments. Leading suppliers offer turnkey installation packages, including network integration, torque calibration, and staff training. Distributors should confirm whether installation is included in the purchase agreement or billed separately, and ensure local technical partners are trained on the specific model.
4. What warranty terms are standard for dental implant motors in 2026, and what do they cover?
Standard warranties range from 2 to 5 years, covering defects in materials and workmanship under normal use. Premium-tier motors often include extended coverage (up to 5 years) with predictive maintenance alerts via IoT-enabled diagnostics. Warranties typically exclude consumables (e.g., burs, sleeves), damage from improper sterilization, or unauthorized repairs. In 2026, many manufacturers offer optional service contracts that cover labor, parts, and preventive maintenance. Distributors should clarify warranty activation procedures (e.g., online registration), regional service center access, and return logistics (RMA process).
| Warranty Tier | Duration | Coverage Highlights |
|---|---|---|
| Standard | 2 years | Mechanical & electronic defects |
| Premium | 3–5 years | Includes calibration, remote diagnostics |
| Service Contract | Renewable annually | Full coverage, priority support |
5. How can clinics and distributors ensure long-term serviceability and parts availability beyond the warranty period?
Long-term serviceability is critical for ROI. Clinics and distributors should prioritize manufacturers with documented parts availability guarantees (typically 7–10 years post-discontinuation). Evaluate the supplier’s service network density, software update roadmap, and backward compatibility with accessories. In 2026, leading brands provide digital service portals for parts ordering, firmware updates, and calibration logs. Distributors are encouraged to stock critical spares and maintain service agreements with OEMs to ensure uninterrupted support for their client clinics. Always request a parts lifecycle statement prior to large-scale procurement.
Need a Quote for Dental Implant Motor?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


