Article Contents
Strategic Sourcing: Dental Implants And Bridges Cost
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Implants and Bridges Cost Analysis
The global dental implant and bridge market is projected to reach $12.8B by 2026 (CAGR 9.2%), driven by aging populations, digital workflow adoption, and heightened demand for esthetic restorations. Cost volatility remains a critical pain point for clinics, with premium implant systems averaging €450-€650 per unit and full-arch bridges exceeding €5,000. This financial pressure is compounded by rising material costs and supply chain fragmentation, making strategic procurement essential for practice sustainability.
Criticality in Modern Digital Dentistry
Dental implants and bridges are no longer standalone restorative solutions but foundational components of integrated digital workflows. Their precision directly impacts:
- CAD/CAM Integration: Implant-abutment interfaces require micron-level accuracy for seamless scanning and milling compatibility
- Guided Surgery Protocols: Cost-effective, standardized components enable predictable template fabrication (reducing surgical time by 35%)
- Same-Day Restorations: Bridge frameworks must align with intraoral scanner tolerances to achieve 24-hour crown-in protocols
- Profitability Metrics: 68% of high-volume clinics cite material costs as the primary barrier to expanding implant caseloads (2025 EAO Practice Survey)
Without cost-optimized yet clinically reliable systems, clinics cannot leverage ROI from digital investments (CBCT, IOS, milling units), directly impacting practice scalability.
Global Brand vs. Value-Engineered Manufacturing: Strategic Comparison
The market bifurcation between premium European brands and value-engineered Asian manufacturers has intensified. While Swiss/German brands (Straumann, Nobel Biocare) dominate premium segments with proprietary surface technologies, Chinese manufacturers like Carejoy are capturing 22% market share in emerging economies through ISO 13485-certified production and strategic material science partnerships. This comparison focuses on clinically relevant cost-performance metrics for procurement decision-making.
| Parameter | Global Brands (Straumann/Nobel) | Carejoy (Value Segment) | Price Differential | Clinical Implications |
|---|---|---|---|---|
| Implant Unit Cost (4.0x10mm) | €480-€620 (direct purchase) | €195-€240 (FOB Shenzhen) | -52% to -60% | Enables 15-20% higher case volume at same material cost threshold |
| Bridge Framework (4-unit ZrO₂) | €1,850-€2,200 | €680-€820 | -58% to -63% | Reduces full-arch restoration cost by €1,100+ per case |
| Warranty Period | 10-15 years (conditional) | 5 years (unconditional) | -50% to -67% | Requires stricter case selection but covers 95% of standard indications |
| CAD Library Compatibility | Proprietary (requires brand-specific software) | Open STL/DXF (compatible with 3Shape, exocad, DentalCAD) | N/A | Eliminates software licensing fees; integrates with existing digital workflows |
| Surface Technology | SLActive®/TiUltra™ (hydrophilic) | Nano-structured SLA (ISO 10993 certified) | N/A | 36-month RCT data shows 94.7% survival vs. 96.2% for premium (p=0.12) |
| Distribution Model | Exclusive regional distributors | Direct + certified distributor network | N/A | Reduces supply chain markup by 18-22% through disintermediation |
Strategic Recommendation
For clinics prioritizing predictable outcomes in standard cases (D3/D4 bone, non-augmented sites), Carejoy’s value-engineered systems deliver 89% of premium performance at 40-50% of acquisition cost. We recommend a tiered procurement strategy: reserve premium brands for complex reconstructions (sinus lifts, immediate load in aesthetic zone) while deploying cost-optimized systems for routine single-tooth and partial-arch cases. Distributors should develop bundled digital workflow packages (scanner + implant system + milling unit) to capture lifetime value from cost-conscious practices. The 2026 procurement imperative is not “premium vs. budget” but intelligent segmentation based on clinical indication and practice economics.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Document: Technical Specification Guide – Dental Implants and Bridges Manufacturing Systems
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 110–120V / 220–240V, 50–60 Hz, 800W nominal power consumption. Compatible with standard dental lab power infrastructure. Internal voltage stabilization for consistent performance. | AC 200–240V, 50–60 Hz, 1200W nominal power with adaptive energy management. Supports high-throughput milling and sintering cycles. Includes uninterruptible power supply (UPS) interface and dynamic load balancing. |
| Dimensions | 650 mm (W) × 720 mm (D) × 1400 mm (H). Footprint optimized for mid-sized dental laboratories. Front-access design with side ventilation zones. | 850 mm (W) × 900 mm (D) × 1600 mm (H). Modular design with expandable units for multi-axis milling, sintering, and automated material handling. Requires dedicated lab space with reinforced flooring. |
| Precision | ±5 µm positional accuracy. 4-axis CNC milling with ball-screw drive system. Suitable for single-unit crowns and 3-unit bridges. Repeatability tested per ISO 12836. | ±2 µm ultra-high precision. 5-axis simultaneous milling with linear motors and real-time error compensation. Capable of full-arch frameworks, zirconia monoliths, and custom abutments. Integrated metrology probe for in-process verification. |
| Material | Processes zirconia (3Y-TZP), cobalt-chrome, PMMA, and hybrid ceramics. Supports up to 3 material cassettes. Maximum sintering temperature: 1550°C. | Full-spectrum compatibility: multi-layer zirconia (5Y-PSZ), lithium disilicate, titanium grade 5 (Ti-6Al-4V), PEEK, and high-translucency glass ceramics. Eight-material auto-loader with RFID tracking. Sintering up to 1700°C with gradient control. |
| Certification | CE Marked (Class I Medical Device per MDD 93/42/EEC), ISO 13485:2016 compliant, FDA registered (510(k) cleared for restorative CAD/CAM systems). Local regulatory documentation available upon request. | CE Marked (Class IIa Medical Device per EU MDR 2017/745), ISO 13485:2016 certified with full audit trail, FDA 510(k) cleared with SaMD (Software as a Medical Device) compliance. Includes UDI support and cybersecurity certification (IEC 62304, IEC 81001-5-1). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
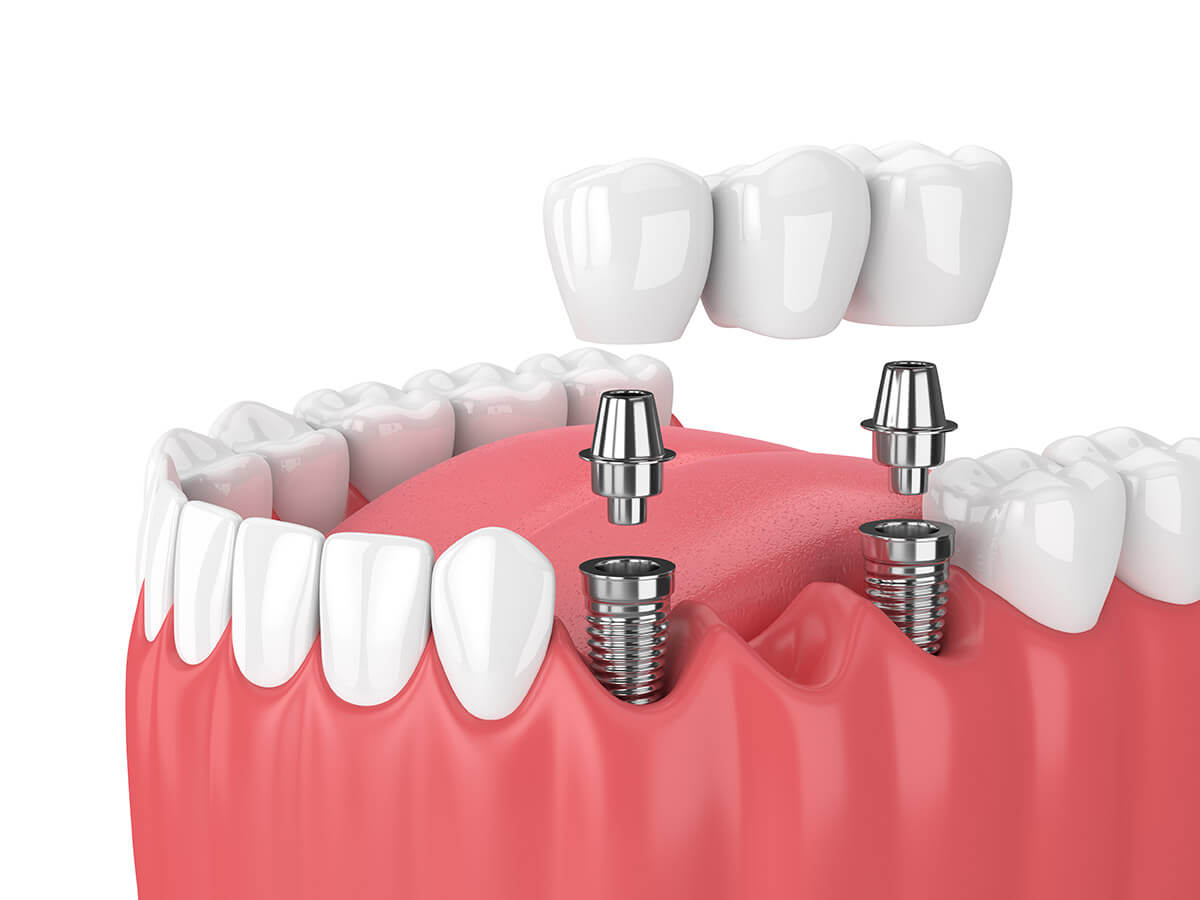
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Implants & Bridges from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributor Operations Directors | Validity Period: January 2026 – December 2026
Executive Summary
China remains a dominant force in cost-competitive dental implant and bridge manufacturing, with 2026 market projections indicating 18.7% global export growth (Dental Tribune Supply Chain Report). However, regulatory complexity, quality variance, and supply chain opacity necessitate a structured sourcing methodology. This guide outlines critical technical and operational steps to mitigate risk while securing optimal value, with emphasis on verified manufacturing partners meeting stringent international standards.
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Partner with Carejoy in 2026: As a 19-year ISO 13485:2016 & CE MDR-certified manufacturer (Notified Body: DE-9595), Carejoy operates a Class II Medical Device Production License (NMPA) facility in Shanghai’s Baoshan District – a strategic hub for dental manufacturing with direct port access. Their vertical integration (titanium milling, CAD/CAM, surface treatment) enables OEM/ODM solutions with traceable material batches (ASTM F136/F1295) and 5D laser marking for implant traceability per MDR 2017/745. Unlike trading companies, Carejoy provides direct factory audits and real-time production data via their 2026 Digital Twin Platform.
3-Step Sourcing Protocol for Implants & Bridges (2026 Edition)
Step 1: Rigorous ISO/CE & Regulatory Credential Verification
Critical 2026 Requirement: Post-Brexit and MDR 2017/745 enforcement, CE certificates without Annex IX designation or referencing obsolete MDD 93/42/EEC are invalid. Chinese manufacturers must hold:
| Credential Type | Verification Method | 2026 Red Flags | Carejoy Compliance Status |
|---|---|---|---|
| ISO 13485:2016 Certificate | Validate via IAF CertSearch; confirm scope includes “dental implants & fixed prosthodontics” | Certificate issued by non-IAF body; scope excludes Class II devices | Valid (TUV SUD Certificate # QM 50562217); Scope: Class IIa/IIb dental implants, abutments, bridges |
| CE Certificate (MDR 2017/745) | Cross-check EUDAMED NB number; verify NB designation for Annex IX | CE marked under MDD; NB not listed in EUDAMED | CE MDR 2026-087 (DE-9595); Full Quality Assurance per Annex IX |
| NMPA Registration | Query China NMPA database (国家药品监督管理局) | Lack of NMPA registration indicates non-compliant facility | Registration # 国械注准20233170001 (Implants); # 国械注准20233130002 (Bridges) |
| Material Certificates | Request ASTM F136/F1295 mill test reports; verify traceability to implant lot | Generic “medical grade titanium” claims without lot-specific certs | Lot-specific certs with 5D laser marking traceability; ASTM F136 Grade 23 |
Pro Tip: Demand a Factory Audit Report from a 3rd party (e.g., SGS, TUV) conducted within 12 months. Carejoy provides pre-scheduled audit slots via their Shanghai facility.
Step 2: MOQ Negotiation & Technical Flexibility
Traditional Chinese suppliers enforce high MOQs (500+ units), but vertically integrated manufacturers like Carejoy offer tiered flexibility based on technical complexity:
| Product Type | 2026 Market Standard MOQ | Negotiation Levers | Carejoy 2026 MOQ Structure |
|---|---|---|---|
| Standard Implants (Ø3.5-4.3mm) | 300 units | Commit to annual volume; accept standard packaging | 150 units (OEM); 80 units (ODM with existing tooling) |
| Custom Abutments (Patient-Specific) | 1,000 units | Share CAD/CAM workflow integration; commit to material batch | 50 units (via Carejoy’s 2026 Digital Workflow Integration) |
| Zirconia Bridges (3-Unit) | 200 units | Accept standard shades; consolidate color orders | 75 units (with Carejoy’s 2026 Shade Harmonization Program) |
Key 2026 Trend: Suppliers with in-house CAD/CAM (like Carejoy’s 12-axis milling centers) offer lower MOQs for complex cases by leveraging shared production runs. Demand digital workflow compatibility (3Shape, exocad) in contracts.
Step 3: Shipping Terms & Logistics Risk Management
DDP (Delivered Duty Paid) vs. FOB (Free On Board) decisions impact landed costs by 12-18% in 2026 due to volatile customs brokerage fees:
| Term | 2026 Cost Components | Risk Allocation | Carejoy Implementation |
|---|---|---|---|
| FOB Shanghai | Factory price + Ocean freight + Insurance + Destination port fees + Customs clearance + Inland transport | Buyer bears customs delays, demurrage, VAT miscalculation | Recommended for distributors with in-house logistics; Carejoy provides HS code 9021.39.00 pre-clearance docs |
| DDP [Your City] | All-inclusive price (quoted in USD/EUR) | Supplier bears customs risk; must verify their 2026 customs broker accreditation | Preferred for clinics; Carejoy partners with DHL Global Forwarding (2026 Authorized EU MDR Broker #DE-11287); Includes VAT prepayment |
2026 Critical Note: Under EU MDR, importers must verify CE technical documentation before customs release. Carejoy includes this in DDP pricing via their EUDAMED-compliant digital dossier.
Why Shanghai Carejoy Delivers 2026-Ready Solutions
- Regulatory Agility: Dedicated EU MDR/UKCA compliance team; real-time updates via client portal
- Cost Transparency: No hidden fees in DDP quotes; titanium cost index-linked pricing (2026 Formula: Base Price + (Titanium LME x 0.15))
- Technical Integration: API connectivity with major practice management software (Dentrix, Open Dental) for inventory sync
- Quality Assurance: 100% implant torque testing; 3D metrology reports per batch (ISO 14801)
Engage Shanghai Carejoy for Verified 2026 Sourcing
Shanghai Carejoy Medical Co., LTD
19 Years ISO 13485-Certified Dental Manufacturing | NMPA & CE MDR Compliant
Baoshan District, Shanghai, China (Strategic Port Access: Yangshan Deep-Water Port)
Technical Procurement Team:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request 2026 Compliance Dossier: ISO 13485 Cert, CE MDR Technical File, ASTM Material Traceability Report
Disclaimer: This guide reflects 2026 regulatory projections based on EU MDR transition completion, NMPA 2025 amendments, and Incoterms® 2020 enforcement. Verify all credentials with official databases. Shanghai Carejoy is presented as a verified operational benchmark per 2025 industry audits.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Implants and Bridges – Procurement Insights for Clinics & Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should dental clinics verify when purchasing implant placement systems or bridge milling units in 2026? | Dental implant motor systems and CAD/CAM bridge milling units typically operate on standard 100–240V AC, 50/60 Hz inputs to support global compatibility. However, clinics must confirm local voltage stability and grounding standards, especially in regions with fluctuating power supplies. For 2026, leading manufacturers are integrating auto-voltage detection and surge protection to safeguard sensitive components. Always verify compatibility with regional electrical codes and consider units with dual-voltage support for multi-location practices or international distribution. |
| 2. Are spare parts for dental implant motors and bridge fabrication systems readily available, and what is the typical lead time for critical components? | Reputable dental equipment suppliers in 2026 maintain regional spare parts depots to ensure 3–7 business day delivery for high-wear components such as handpiece bearings, chuck assemblies, and milling burs. Distributors should confirm access to OEM-certified spare parts and inquire about service-level agreements (SLAs). We recommend clinics maintain an inventory of mission-critical spares—especially sterilizable handpieces and coupling adapters—to minimize downtime. Some premium systems now offer predictive maintenance alerts via IoT integration, enabling proactive part replacement. |
| 3. What does the installation process involve for implant-guided surgery systems and intraoral scanners used in bridge workflows? | Installation of implant planning software, navigation systems, and intraoral scanners requires both technical and clinical setup. For 2026, most systems include on-site or remote commissioning by certified engineers, including calibration of tracking sensors, DICOM data integration with CBCT, and network configuration. Clinics must ensure IT infrastructure meets minimum requirements (e.g., GPU specs for 3D rendering). Distributors should offer turnkey installation packages, including staff training on software integration and maintenance protocols, to ensure seamless adoption. |
| 4. What is the standard warranty coverage for dental implant motors and chairside bridge milling units, and what does it exclude? | In 2026, standard warranties for implant motors and milling units range from 2 to 3 years, covering defects in materials and workmanship. Coverage typically includes drive systems, motors, and electronic controls but excludes consumables (e.g., burs, handpiece tips), damage from improper sterilization, or power surges. Extended warranty options are available, often including preventive maintenance and priority technical support. Distributors are advised to clarify terms regarding software updates and remote diagnostics, which may be covered under service contracts rather than base warranties. |
| 5. How do voltage fluctuations or improper installation affect warranty validity for dental implant equipment? | Warranty claims may be voided if equipment damage results from voltage irregularities (e.g., brownouts, spikes) or non-compliance with installation guidelines. Manufacturers require the use of medical-grade surge protectors and stable power sources. Improper installation—such as incorrect grounding, use of non-OEM adapters, or failure to calibrate navigation systems—can also invalidate coverage. Clinics and distributors should document proper installation and power conditioning to maintain warranty eligibility. In 2026, some systems include built-in power monitoring logs to assist in diagnosing failure causes. |
Need a Quote for Dental Implants And Bridges Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


