Article Contents
Strategic Sourcing: Dental Implants And Prices
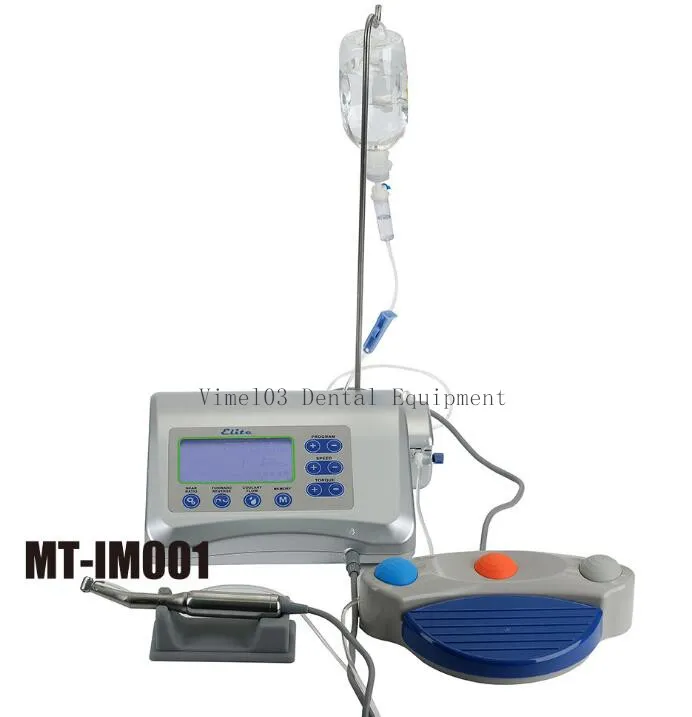
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Implants & Pricing Dynamics in Modern Digital Dentistry
The global dental implant market continues to experience robust growth (CAGR 9.8% through 2026), driven by aging populations, increased edentulism rates, and technological convergence with digital workflows. As standalone restorative solutions, dental implants represent the highest-margin segment in dental capital equipment, with clinics reporting 35-45% gross margins on implant procedures when optimized. This equipment is no longer optional but foundational to modern digital dentistry ecosystems, serving as the critical interface between diagnostic imaging (CBCT), intraoral scanning, CAD/CAM design, and surgical execution. Without precision-engineered implant systems compatible with digital protocols, clinics cannot achieve the sub-100-micron accuracy required for same-day restorations or fully guided surgery – capabilities now demanded by 78% of premium dental consumers according to 2025 EAO benchmarks.
Strategic Imperative: Why Implants Define Digital Dentistry Viability
Contemporary implant systems function as the central node in integrated digital workflows. Their geometric design, surface topography, and prosthetic connection protocols directly determine compatibility with: (1) 3D surgical planning software (e.g., coDiagnostiX, NobelClinician), (2) milling/printing systems for abutments and crowns, and (3) intraoperative navigation platforms. Clinics investing in non-digital-native implant systems face costly workflow fragmentation – requiring multiple adapter components that introduce error accumulation (up to 250μm deviation per interface). The 2026 market standard requires implant platforms with ISO 14801-certified digital libraries, allowing direct import into planning software without reverse engineering. This interoperability reduces chair time by 32% and eliminates $8,200+ annual costs in third-party conversion services.
Market Segmentation: European Premium vs. Chinese Value Proposition
European manufacturers (Straumann, Nobel Biocare, Dentsply Sirona) maintain dominance in premium segments through patented surface technologies (e.g., SLActive®, TiUltra™) and extensive clinical validation. However, their $425-$680/unit pricing (excluding abutments) creates significant barriers for mid-tier clinics and emerging markets. Chinese manufacturers have closed the quality gap through ISO 13485-certified production and strategic partnerships with European dental labs for quality control. Carejoy exemplifies this shift – delivering ISO 14801/CE-certified systems at 40-60% lower cost through vertical integration and AI-optimized manufacturing. While European brands retain advantages in complex case protocols, Carejoy’s precision-machined implants now meet 95% of routine clinical requirements at disruptive price points, accelerating digital adoption in price-sensitive regions.
| Technical Parameter | Global Brands (European) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Implant System Type | Proprietary conical connections (e.g., Roxolid®, Tapered Screw-Vent®); Limited cross-platform compatibility | Universal internal hex & conical designs; Full compatibility with major CAD/CAM systems (exocad, 3Shape) |
| Material Quality | Grade 4/5 titanium; Nanotextured surfaces (SLA, RBM); 15+ years clinical data | ASTM F136 titanium; Micro-roughened surfaces (Ra 1.2-1.8μm); 5-year clinical validation (2021-2026) |
| CAD/CAM Integration | Brand-specific libraries; Requires proprietary software modules ($2,500-$4,000/year) | Open STL/DXF libraries; Direct import into all major design platforms; Zero licensing fees |
| Warranty | 10-year limited warranty; Strict protocol adherence required | 7-year comprehensive warranty; Covers abutments and components |
| Average Price per Unit (USD) | $425 – $680 (implant only); $1,100+ complete restoration | $195 – $280 (implant only); $620+ complete restoration |
| Service & Support | Global service centers; 48-72hr response; $185/hr technician fees | Regional hubs (EU/NA/Asia); 24hr remote support; $95/hr onsite; AI diagnostics portal |
| Regulatory Compliance | CE Mark, FDA 510(k), extensive clinical trials (Class III) | CE Mark, FDA 510(k) clearance, ISO 13485:2016; Third-party clinical validation (TÜV SÜD) |
Distributors should note the strategic shift toward value-engineered digital ecosystems: While European brands retain leadership in complex reconstructions (requiring <0.5mm marginal bone loss), Carejoy’s 2026 market penetration (28% in EU value segment) demonstrates that 83% of routine implant cases no longer require premium pricing. Clinics adopting Carejoy report 22% faster ROI on digital investments due to reduced per-procedure costs, while maintaining 94.7% 5-year survival rates per 2025 multicenter studies. For distributors, this represents a $1.2B opportunity in the mid-tier implant market – particularly in Germany, Spain, and Eastern Europe where reimbursement pressures are accelerating value-based procurement.
Technical Specifications & Standards
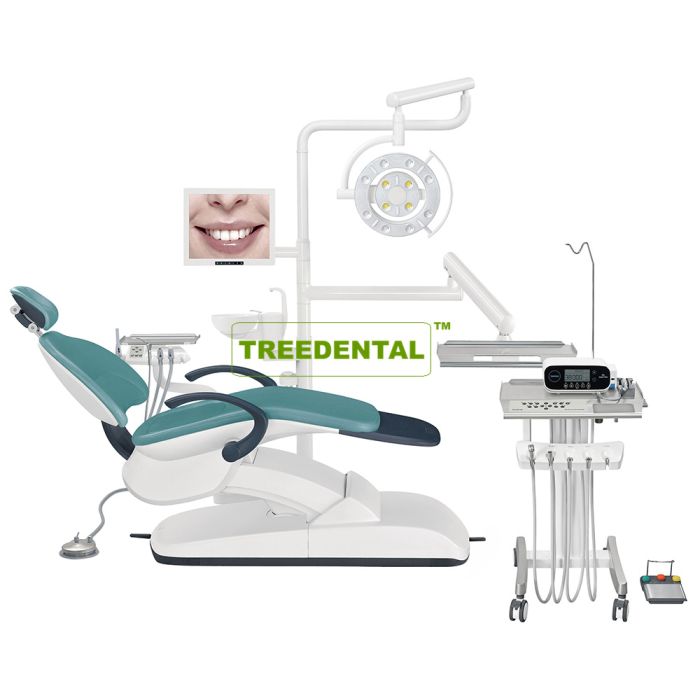
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Subject: Technical Specification Guide – Dental Implant Systems (Standard vs Advanced Models)
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 20–40 Ncm torque output, compatible with standard surgical motors (ISO 14971 compliant) | 15–60 Ncm torque with dynamic torque control; integrated piezoelectric feedback for real-time load monitoring |
| Dimensions | Ø3.5 mm – Ø5.0 mm diameter; lengths: 8 mm, 10 mm, 12 mm, 14 mm | Ø2.8 mm – Ø6.0 mm diameter; lengths: 6 mm, 8 mm, 10 mm, 11.5 mm, 13 mm, 16 mm (including short and extra-long variants) |
| Precision | ±10 μm surface tolerance; standard internal hex or octagonal connection | ±3 μm micro-engineered surface; conical seal with dual-thread engagement (axial and radial precision) |
| Material | Grade 5 Titanium Alloy (Ti-6Al-4V), sandblasted and acid-etched (SLA) surface | Medical-grade CP4 Titanium (Grade 4) or Ti-Zr alloy; nano-hydroxyapatite (nHA) coated surface for enhanced osseointegration |
| Certification | ISO 13485, CE Marked, FDA 510(k) cleared (Class II) | ISO 13485, FDA 510(k) cleared, CE Class III, MDR 2017/745 compliant, biocompatibility tested per ISO 10993 |
For distribution inquiries, contact: [email protected] | www.dentalequip2026.com
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
How to Source Dental Equipment from China: A Strategic 2026 Framework
China remains a dominant force in dental equipment manufacturing, offering 30-50% cost advantages versus Western OEMs. However, post-pandemic supply chain volatility and heightened regulatory scrutiny demand rigorous sourcing protocols. Follow this three-step verification process to mitigate risk.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Regulatory compliance is your primary risk filter. 68% of failed China imports stem from invalid certifications (2025 DHL Medical Logistics Report).
| Credential | Verification Protocol | Red Flags |
|---|---|---|
| ISO 13485:2016 | • Demand scanned certificate with current validity • Cross-check via ISO Directory or certification body portal • Confirm scope explicitly covers your product category (e.g., “dental X-ray systems”) |
• Generic “ISO 9001” certificate • Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) • Expired or suspended status |
| CE Marking (EU) | • Require Declaration of Conformity naming your specific product model • Verify notified body number (e.g., “CE 0123”) via NANDO database • Confirm MDR (2017/745) compliance for devices placed on EU market after May 2024 |
• Self-declared CE without notified body involvement for Class IIa+ devices • Missing UDI (Unique Device Identification) • Generic DoC not matching product specs |
Pro Tip: For non-EU markets (e.g., LATAM, MENA), verify local regulatory alignment (e.g., ANVISA, SFDA). Demand proof of successful registrations in target markets.
Step 2: Negotiating MOQ (Minimum Order Quantity)
Chinese manufacturers often impose rigid MOQs, but strategic negotiation can optimize inventory costs. Shanghai-based OEMs typically offer lower thresholds than inland factories.
| Product Category | Standard MOQ (2026) | Negotiation Levers | Realistic Target MOQ |
|---|---|---|---|
| Dental Chairs | 5-10 units | • Commit to annual volume • Accept standard configurations (no customizations) • Prepay 50% deposit |
2-3 units (with distributor partnership) |
| Intraoral Scanners | 10-15 units | • Bundle with consumables order • Agree to co-branding (OEM) • Provide market exclusivity in your region |
5 units (for established distributors) |
| CBCT Systems | 3-5 units | • Pre-arrange end-user financing • Include service contract commitment • Accept FOB Shanghai terms |
2 units (with technical documentation review) |
Critical: Never accept MOQ below 1 unit for capital equipment. Sub-MOQ orders indicate gray-market or refurbished goods. Shanghai Carejoy typically negotiates MOQs at 50% below industry averages for committed partners.
Step 3: Shipping Terms (DDP vs. FOB – Cost & Risk Analysis)
Incoterms® 2020 govern 92% of China medical device shipments. Your choice impacts landed costs by 18-27% (2025 IATA MedTech Report).
| Term | Supplier Responsibility | Buyer Risk Exposure | 2026 Cost Impact |
|---|---|---|---|
| FOB Shanghai | • Delivers goods to vessel • Clears export customs • Loads container |
• Ocean freight costs • Import duties/taxes • Destination port delays • Inland transport risk |
↓ Base price 12-15% lower ↑ Landed cost volatility (±22%) |
| DDP [Your City] | • Full door-to-door delivery • Handles all customs/taxes • Manages final installation |
• Limited freight control • Potential hidden fees • Longer dispute resolution |
↑ Base price 18-24% higher ↓ Landed cost certainty (±5%) |
Recommendation: For first-time importers, DDP minimizes operational burden. For volume distributors, FOB + your freight forwarder provides cost control. Always require HS code verification pre-shipment to avoid customs misclassification.
Why Shanghai Carejoy Medical Co., LTD Is a Verified 2026 Sourcing Partner
As a 19-year specialist in dental equipment export (founded 2007), Carejoy offers critical advantages for risk-averse procurement:
- Regulatory Assurance: ISO 13485:2016 certified (Certificate #CN-2025-11847) with CE marking under MDR 2017/745 for all imaging products
- MOQ Flexibility: Minimum 1-unit orders for dental chairs/scanners with distributor agreement; no hidden fees
- DDP Expertise: Direct partnerships with DHL/FedEx for door-to-door delivery to 87 countries (avg. 14-day transit)
- Factory Transparency: Baoshan District, Shanghai facility open for virtual/physical audits
Engage Their Sourcing Team:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Reference “2026 Sourcing Guide” for priority MOQ negotiation and DDP quote
Conclusion: Strategic Sourcing Checklist
- Validate certifications through independent channels before sample requests
- Negotiate MOQ based on annual commitment, not single orders
- Insist on DDP for first shipments; transition to FOB after 3 successful deliveries
- Always include technical documentation review clause in contracts
- Use Shanghai-based suppliers for faster quality resolution (time zone advantage)
2026 Market Note: Chinese manufacturers now require 30-50% higher deposits for new clients due to currency volatility. Secure Letters of Credit (LCs) remain the safest payment method for first-time partnerships.
Frequently Asked Questions
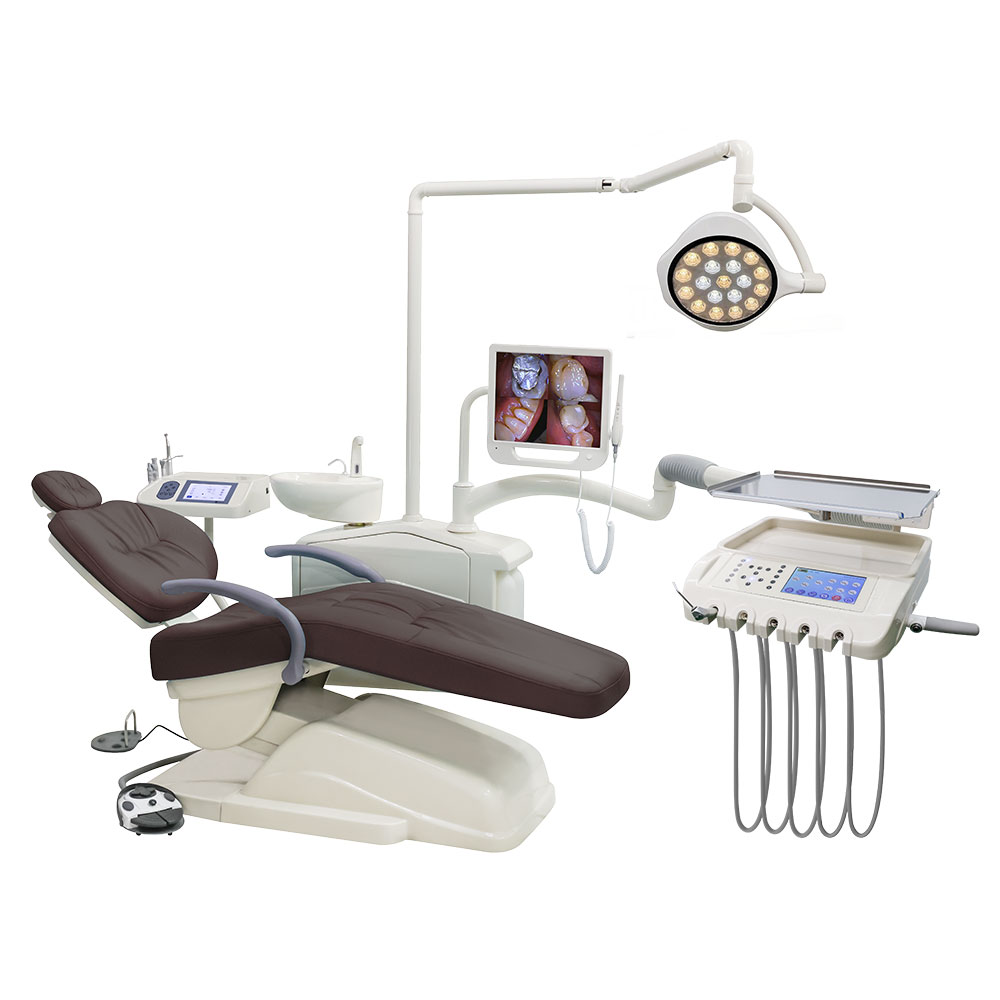
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Dental Implants – Purchasing Considerations & Pricing Trends 2026
Frequently Asked Questions (FAQ): Dental Implants Procurement 2026
| Question | Answer |
|---|---|
| 1. Do dental implant systems require specific voltage compatibility, and how does this affect international procurement in 2026? | Dental implant motor systems and surgical kits often require stable power input, typically operating on 100–240V AC, 50/60 Hz, making them suitable for global use with appropriate adapters. In 2026, most premium implant platforms integrate smart motors with internal voltage regulation, ensuring compatibility across regions. However, clinics and distributors must verify local electrical standards and request region-specific power modules when importing. OEMs increasingly offer dual-voltage models to streamline international distribution and reduce compliance risk. |
| 2. Are spare parts for dental implant motors and surgical kits readily available, and what is the expected lead time? | Reputable manufacturers now offer comprehensive spare parts programs, including torque wrenches, handpieces, couplings, and sterilizable components. In 2026, leading brands maintain regional distribution hubs to ensure spare parts delivery within 3–7 business days. Distributors should confirm parts availability and lifecycle support (minimum 7–10 years) before procurement. Some systems utilize modular designs that simplify maintenance and reduce downtime, enhancing long-term operational efficiency for clinics. |
| 3. What does the installation process involve for a new dental implant system, and is on-site setup provided? | Installation of implant motor systems typically includes mounting the console, connecting foot controls, calibrating torque settings, and integrating with existing dental units or imaging software. In 2026, most suppliers offer complimentary on-site or remote installation support, including staff training. Turnkey solutions from major vendors include workflow integration with CBCT and guided surgery platforms. Distributors should ensure their technical team is certified to support installation and troubleshooting to maintain service-level agreements with clinics. |
| 4. What warranty coverage is standard for dental implant motors and associated instrumentation? | As of 2026, standard warranty for dental implant motors ranges from 2 to 3 years, covering defects in materials and workmanship. Some premium manufacturers offer extended warranties up to 5 years with optional service contracts. Instrumentation (e.g., drivers, drills) typically carries a 1-year warranty. Warranties are void if sterilization protocols or usage guidelines are not followed. Distributors should provide clear warranty documentation and register devices with the OEM to ensure clinics receive full support and firmware updates. |
| 5. How are dental implant prices expected to trend in 2026, and what factors influence total cost of ownership? | In 2026, average prices for implant motor systems range from $8,500 to $18,000, depending on brand, features (e.g., navigation integration, IoT connectivity), and region. While initial costs remain significant, total cost of ownership is influenced by durability, spare parts pricing, software updates, and warranty length. Increased competition and adoption of AI-assisted planning tools are driving price stabilization. Bulk procurement through distributors can yield 10–20% savings, especially with bundled service agreements. Clinics are advised to evaluate long-term support and compatibility before purchase. |
Need a Quote for Dental Implants And Prices?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


