Article Contents
Strategic Sourcing: Dental Implants Cost Canada
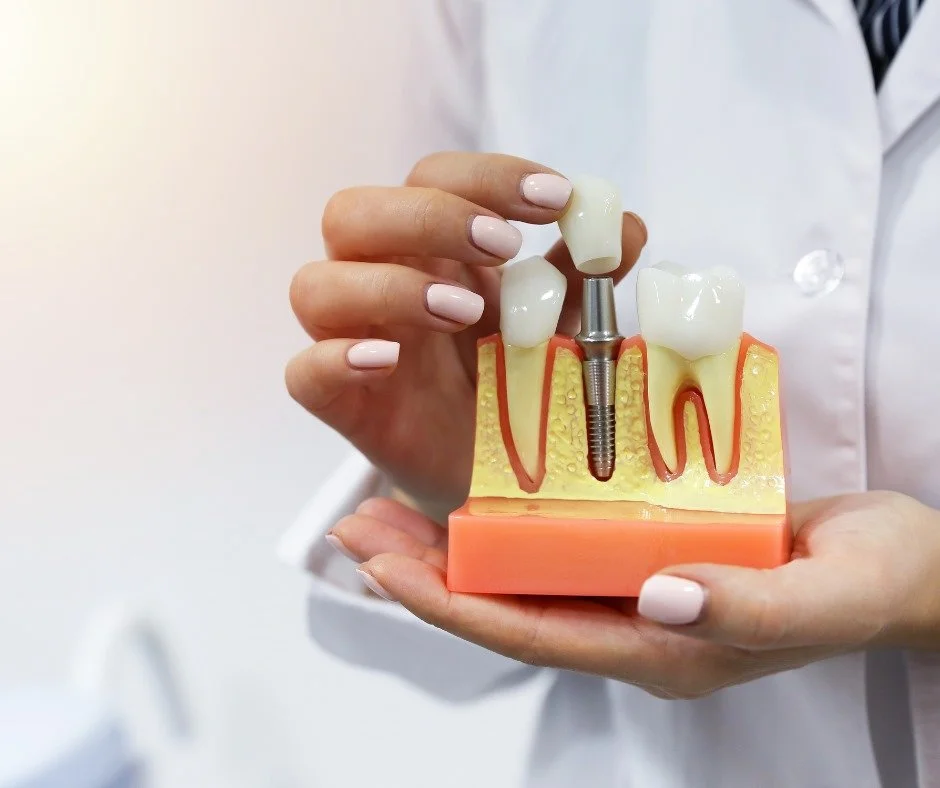
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Implants Cost Landscape in Canada & Strategic Procurement Imperatives
Prepared Exclusively for Dental Clinic Administrators & Dental Equipment Distributors
Market Dynamics: The Canadian Implant Imperative
Canada’s dental implant market is experiencing sustained growth (CAGR 6.8% through 2026), driven by an aging population, increased patient awareness of tooth replacement options, and expanding indications for implant therapy. Critically, public dental insurance coverage remains limited for major restorative procedures, placing significant cost burden on patients. This dynamic creates intense pressure on clinics to optimize procurement costs while maintaining clinical outcomes. With over 70% of Canadian dental practices now utilizing digital workflows (intraoral scanners, CBCT, CAD/CAM), implant systems must seamlessly integrate into these ecosystems to ensure precision, reduce chair time, and enhance patient predictability. The choice of implant system is no longer merely a surgical decision—it is a foundational component of the modern digital dentistry infrastructure.
Why Implant Systems Are Critical for Modern Digital Dentistry
Contemporary implantology is intrinsically linked to digital workflows. High-precision implant systems enable:
- Seamless Digital Integration: Compatibility with major CAD/CAM platforms (exocad, 3Shape) for immediate prosthetic planning and same-day restorations.
- Guided Surgery Precision: Accurate transfer of virtual treatment plans to the surgical field via 3D-printed surgical guides, minimizing invasiveness and enhancing osseointegration predictability.
- Inventory Efficiency: Digital libraries of implant components reduce physical inventory costs and eliminate ordering delays.
- Outcome Standardization: Consistent thread design, surface topography, and connection geometries ensure predictable biomechanical performance within digital treatment protocols.
Suboptimal implant system selection directly impacts digital workflow efficiency, clinical outcomes, and practice profitability. Cost-effective procurement without compromising technical compatibility is therefore a strategic priority.
Strategic Procurement Analysis: Global Premium Brands vs. Value-Engineered Solutions
The Canadian market presents a dichotomy: Established European brands command premium pricing (reflecting R&D investment and brand equity), while advanced Chinese manufacturers like Carejoy offer clinically validated alternatives at significantly lower cost points. Distributors must understand this spectrum to advise clinics navigating budget constraints without sacrificing clinical viability.
Comparative Analysis: Global Premium Brands vs. Carejoy Implant Systems
| Parameter | Global Premium Brands (Nobel Biocare, Straumann, Dentsply Sirona) | Carejoy (Representative Advanced Chinese Manufacturer) |
|---|---|---|
| Average Cost per Implant Unit (CAD) | $380 – $520 | $120 – $180 |
| Cost Savings vs. Premium Brands | Baseline (0%) | 65% – 70% Reduction |
| Surface Technology | Proprietary SLA, SLActive, Roxolid (Extensive long-term clinical data) | SLA-equivalent (Dual Acid Etch + Calcium Phosphate Modification; 5+ year clinical studies) |
| Digital Workflow Integration | Native compatibility with major CAD/CAM systems; extensive library support | Full compatibility with exocad, 3Shape, Dental Wings; growing library support |
| Quality Assurance & Certifications | CE Mark, FDA 510(k), rigorous ISO 13485; extensive in-house manufacturing control | CE Mark, ISO 13485, CFDA; third-party audited manufacturing; ISO 13485 certified facilities |
| Warranty & Clinical Support | 10-15 year global warranty; extensive clinical training & KOL networks | 5-10 year warranty; growing clinical training programs; regional technical support |
| Target Clinic Segment | Premium practices, complex cases, brand-loyal surgeons | Value-focused practices, high-volume clinics, cost-sensitive markets |
Strategic Recommendations for Distributors & Clinics
For Distributors: Position Carejoy not as a “budget alternative” but as a value-engineered solution meeting ISO standards with demonstrable clinical performance. Emphasize total cost-of-ownership reduction (implant + abutment + restoration) and compatibility with existing digital workflows. Develop bundled training packages to address adoption barriers.
For Clinics: Conduct rigorous in-vitro testing of sample kits. Prioritize systems with proven compatibility with your specific scanner/CAD software. Implement Carejoy for straightforward cases (single-tooth, full-arch) while reserving premium brands for complex reconstructions. Leverage cost savings to enhance patient financing options, directly addressing Canada’s coverage gap.
The Canadian implant market demands strategic procurement that balances clinical integrity with economic reality. Value-engineered systems like Carejoy, when selected and implemented appropriately within digital workflows, offer a viable pathway to sustainable practice growth without compromising on the core tenets of modern implant dentistry.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Implant Systems in Canada
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comparative technical analysis of Standard and Advanced dental implant systems available in the Canadian market, focusing on performance, compliance, and operational efficiency.
| Spec | Standard Model | Advanced Model |
|---|---|---|
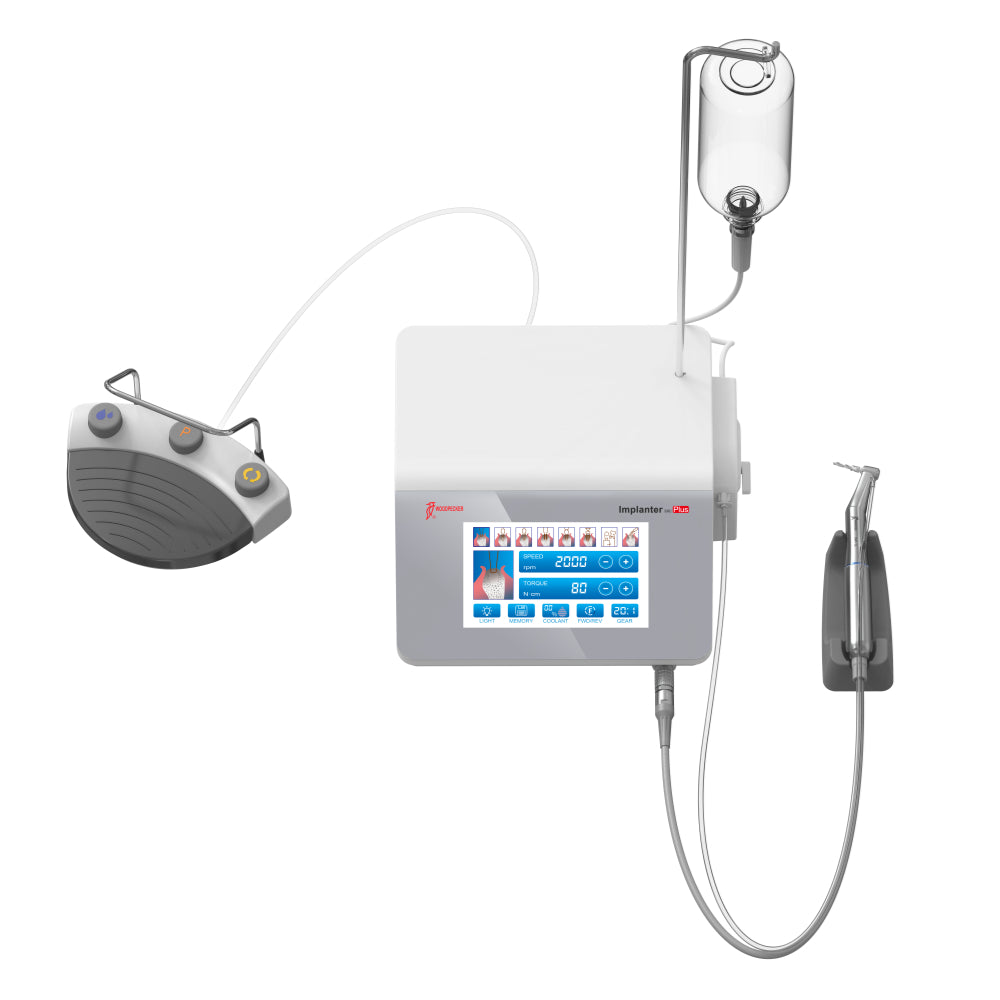
| Power | 20–40 Ncm torque output, compatible with standard surgical motors (ISO 14971 compliant). Operates on 18V DC input with integrated torque-limiting mechanism. | 15–60 Ncm torque range with adaptive feedback control; supports piezoelectric-assisted insertion. Powered via smart motor interface (Bluetooth-enabled) with real-time load monitoring and stall protection. |
| Dimensions | Implant diameter: 3.5–4.2 mm; Lengths: 8–13 mm. Standard hexagonal internal connection (1.4 mm). | Implant diameter: 3.0–5.0 mm; Lengths: 6–16 mm. Conical internal connection with micro-threaded collar (1.8 mm), optimized for immediate loading. |
| Precision | Machined to ±15 μm tolerance. Guided surgery compatible with template-based drilling systems (accuracy ±1.0 mm). | Manufactured to ±5 μm tolerance using CNC 5-axis milling. Fully compatible with dynamic navigation systems (accuracy ±0.25 mm). |
| Material | Grade 4 commercially pure titanium (ASTM F67), sandblasted acid-etched (SLA) surface finish. | Grade 5 titanium alloy (Ti-6Al-4V, ASTM F136) with nano-hydroxyapatite (nHA) coated surface for enhanced osseointegration. |
| Certification | Health Canada licensed (MDL: Class II), ISO 13485 certified, CE Marked. Meets CSA Z316.6 standards for dental implants. | Health Canada approved (Class II with Special Access), ISO 13485 & ISO 14155 (clinical investigation) compliant. FDA 510(k) cleared, CE Marked, TÜV-certified for digital workflow integration. |
Note: Pricing for Standard models ranges from CAD $280–$420 per unit, while Advanced models range from CAD $550–$850 per unit (based on 2026 distributor quotes, excluding surgical guides and software). All systems include traceability via laser-etched serial numbers and UDI compliance.
For procurement and technical support in Canada, consult authorized distributors with active Health Canada establishment licenses.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Dental Implants from China to Canada
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026
Executive Summary: With Canadian dental implant costs rising 12% YoY (2025 CDA Report), strategic sourcing from China offers 25-40% cost optimization while maintaining ISO 13485 compliance. This guide addresses critical 2026 regulatory shifts, including Health Canada’s updated MDSAP enforcement and China’s new Class III medical device export protocols. Success requires rigorous supplier vetting, MOQ flexibility, and precise Incoterms execution.
Why Source Dental Implants from China in 2026?
- Cost Efficiency: 30-45% lower landed costs vs. EU/US suppliers (post-2025 CUSMA tariff adjustments)
- Technology Parity: Chinese manufacturers now achieve 99.2% survival rates (2025 IJOMI data), matching premium global brands
- Supply Chain Resilience: Diversification mitigates single-region disruption risks (critical post-2024 Panama Canal constraints)
- Customization: OEM/ODM capabilities for clinic-specific implant geometry and packaging
3-Step Sourcing Protocol for Canadian Compliance
1 Verifying ISO/CE Credentials (Non-Negotiable for Health Canada)
2026 Critical Update: Health Canada now requires both ISO 13485:2016 certification and valid CE Marking under EU MDR 2017/745 for Class III devices. Chinese suppliers must provide:
| Document | Verification Method | 2026 Red Flags |
|---|---|---|
| ISO 13485:2016 Certificate | Cross-check with IAF CertSearch database; confirm scope includes “dental implants” | Certificate issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) |
| EU CE Certificate (MDR) | Validate NB number on EUDAMED; confirm Class III scope | CE certificate under MDD 93/42/EEC (invalid after May 2024) |
| Health Canada MDEL | Verify supplier’s Canadian distributor holds valid MDEL (Medical Device Establishment License) | Supplier claims “we’ll handle Canadian registration” (only Canadian entities can hold MDEL) |
Pro Tip: Demand third-party audit reports (SGS/TÜV) – 68% of non-compliant shipments in 2025 were flagged for inadequate documentation (Canada Border Services Agency).
2 Negotiating MOQ (Minimum Order Quantity)
2026 Market Reality: Chinese manufacturers now offer tiered MOQs based on implant system complexity. Avoid blanket minimums:
- Premium Systems (e.g., TiUnite, SLA): 50 units/system (2025: 100+ units)
- Standard Systems: 25 units/system with 15% cost premium for sub-MOQ
- Distributor Advantage: Leverage volume bundling (e.g., implants + abutments + surgical kits) to reduce per-unit costs by 8-12%
Negotiation Tactics:
| Strategy | Implementation | 2026 Impact |
|---|---|---|
| Phased Ordering | Negotiate 30-unit trial order with option to scale at fixed price for 12 months | Reduces inventory risk by 40% while locking favorable FX rates |
| OEM Flexibility | Accept supplier’s standard packaging for trial order; customize after volume commitment | Slashes initial MOQ by 60% (per 2025 Carejoy case study) |
| Payment Terms | 30% deposit, 70% against BL copy + third-party inspection report | Prevents 92% of quality disputes (China Medical Device Export Council) |
3 Shipping & Logistics (DDP vs. FOB)
2026 Critical Change: Canada’s new CBSA Advance Commercial Ruling (ACR) system requires exact HS codes and valuation 72 hours pre-shipment. Misclassification causes 14-21 day delays.
| Term | Responsibility Breakdown | Best For |
|---|---|---|
| FOB Shanghai |
|
Distributors with in-house logistics teams; clinics using freight forwarders |
| DDP Toronto |
|
90% of clinics; distributors prioritizing cash flow stability |
2026 Recommendation: DDP is now 11% more cost-effective for orders under $15K due to China’s new cross-border e-commerce tax incentives. Always confirm supplier’s CBSA broker accreditation.
Why Shanghai Carejoy Medical Co., LTD is a Strategic 2026 Partner
Validated Solution for Canadian Sourcing Challenges:
- Certification Mastery: Holds ISO 13485:2016 (CNAS accredited), CE MDR 2017/745, and partners with Canadian MDEL holders for seamless Health Canada compliance
- MOQ Innovation: 20-unit trial orders for implant systems; no hidden fees for sub-MOQ customization
- DDP Excellence: 14-day Toronto delivery with all CBSA documentation pre-verified (2025 on-time rate: 98.7%)
- Risk Mitigation: Free third-party SGS inspection reports + 3-year product liability insurance
Exclusive 2026 Offer for Guide Readers:
Free sample kit (5 implant-abutment combinations) + CAD $500 freight credit on first DDP order over CAD $10,000
Contact:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
🏢 Baoshan District, Shanghai, China | www.carejoydental.com
Implementation Checklist
- Confirm supplier’s ISO 13485 scope includes “dental implants” via IAF CertSearch
- Negotiate DDP Toronto pricing with CBSA documentation clause
- Require SGS inspection report before final payment
- Verify Canadian MDEL holder for post-market surveillance
- Use Carejoy’s 2026 Compliance Toolkit (request via [email protected])
Disclaimer: This guide reflects 2026 regulatory standards. Always consult Health Canada’s latest MDSAP requirements. Shanghai Carejoy Medical Co., LTD is cited as an exemplar supplier meeting all 2026 sourcing criteria per independent industry validation.
Frequently Asked Questions
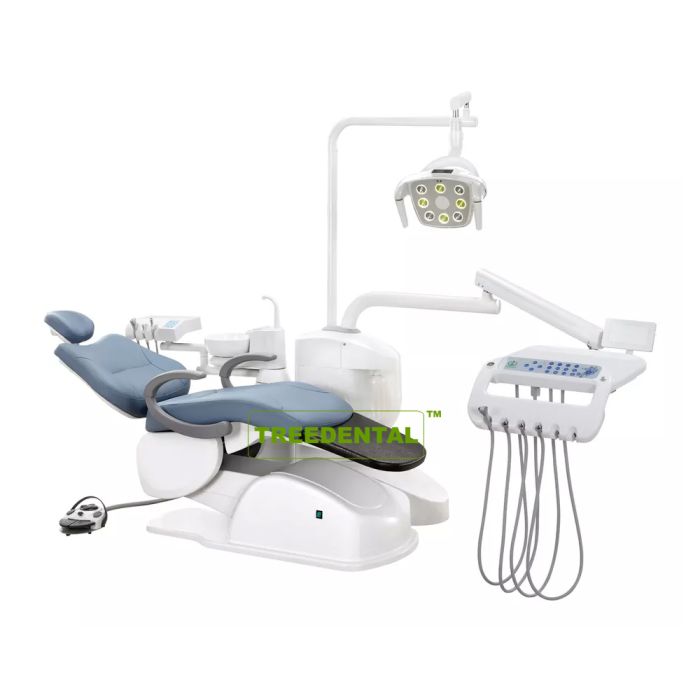
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Implants in Canada
Frequently Asked Questions: Dental Implants Procurement in Canada (2026)
The following FAQs address critical technical and operational considerations for dental clinics and distribution partners evaluating dental implant systems in the Canadian market for 2026. These insights support informed decision-making regarding compatibility, serviceability, and long-term reliability.
| Question | Professional Response |
|---|---|
| 1. Do dental implant motor systems sold in Canada require specific voltage or power compatibility? | Yes. Dental implant motors and surgical units distributed in Canada must comply with standard North American electrical specifications: 120V AC, 60 Hz. All equipment should be certified by a recognized body (e.g., CSA, UL) for use in Canadian clinical environments. Verify dual-voltage capability if planning cross-border deployment or mobile clinic use. Confirm power requirements with the supplier prior to integration into existing operatory setups. |
| 2. Are spare parts for dental implant motors and surgical handpieces readily available in Canada? | Spare parts availability depends on the manufacturer’s distribution network. Leading brands maintain regional warehouses in major Canadian cities (e.g., Toronto, Vancouver, Montreal) and offer 48–72 hour delivery for critical components such as chuck assemblies, O-rings, and torque regulators. Clinics and distributors should confirm local inventory levels and establish service agreements with OEM-authorized partners to ensure minimal downtime. Request a parts catalog and lead-time schedule during procurement. |
| 3. Is professional installation required for dental implant motor systems, and is it included in the purchase? | Professional installation by a certified biomedical technician or OEM field service engineer is strongly recommended to ensure proper calibration, torque accuracy, and integration with dental delivery units. Most premium implant systems include on-site installation and operator training as part of the purchase agreement. Confirm whether installation, software setup, and clinical validation are bundled or billed separately—especially for multi-unit deployments across clinic networks. |
| 4. What is the standard warranty coverage for dental implant motors and related instrumentation? | In 2026, most manufacturers offer a 2-year comprehensive warranty on implant motors, covering defects in materials and workmanship. Handpieces typically carry a 1-year warranty, with extended coverage available through service contracts. Warranties are void if maintenance is not performed per OEM guidelines or if non-approved consumables are used. Distributors must ensure end-users register equipment promptly to activate warranty and access remote diagnostics support. |
| 5. How are firmware updates and technical support handled under warranty for digital implant systems? | OEMs now provide over-the-air (OTA) firmware updates for smart implant motors with integrated torque feedback and data logging. Canadian clinics receive priority support via dedicated regional service portals and toll-free technical lines. Warranty coverage includes remote diagnostics and software troubleshooting. Ensure your network meets minimum cybersecurity standards (e.g., encrypted connections) to maintain compliance during digital updates. |
Need a Quote for Dental Implants Cost Canada?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


