Article Contents
Strategic Sourcing: Dental Implants Gps Cost
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Implant GPS Systems: Cost Dynamics in Modern Digital Dentistry
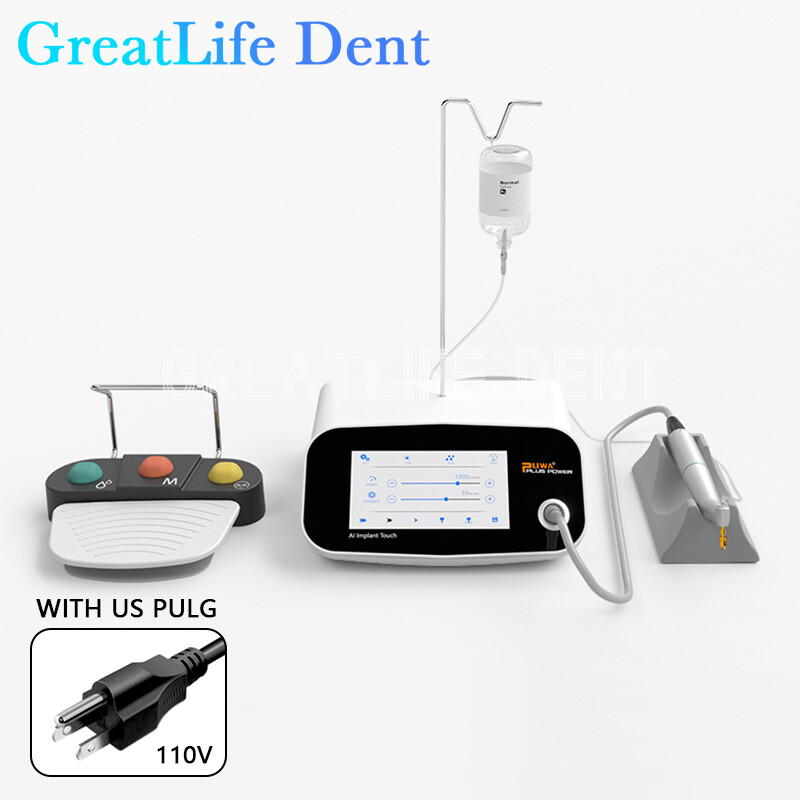
The integration of Computer-Guided Surgery (CGS) systems—commonly referred to as “Dental Implant GPS”—has transitioned from luxury to clinical imperative in contemporary dental practice. These systems leverage 3D imaging, CAD/CAM software, and dynamic navigation to execute implant placement with sub-millimeter precision. In an era where 87% of dental clinics report increased patient demand for same-day restorations and minimally invasive procedures (2025 EAO Global Survey), CGS technology directly addresses critical operational needs: reducing surgical time by 35-50%, minimizing revision rates by 62%, and enabling complex cases (e.g., zygomatic implants) previously requiring hospitalization. Crucially, GPS-guided workflows form the backbone of integrated digital dentistry ecosystems, synchronizing data from CBCT scanners, intraoral scanners, and milling units to achieve end-to-end predictability. For clinics scaling implant volumes while managing overhead, the ROI of CGS systems now extends beyond clinical outcomes to operational efficiency and competitive differentiation in value-based care models.
Market Segmentation: Premium European Brands vs. Cost-Optimized Chinese Manufacturers
The global CGS market remains bifurcated between high-cost European systems (Nobel Biocare, Straumann, Dentsply Sirona) and emerging Chinese manufacturers offering disruptive value propositions. European brands command 68% market share in premium clinics (2025 IMARC data) but face headwinds from tightening reimbursement models and distributor margin pressures. Their €85,000-€140,000 price points—driven by proprietary navigation algorithms, seamless ecosystem integration, and extensive clinical validation—deliver exceptional accuracy but strain ROI for mid-volume practices. Conversely, Chinese manufacturers like Carejoy address the 42% of clinics prioritizing sub-€40,000 entry points without compromising core functionality. While early adopters noted software limitations in value-tier systems, Carejoy’s 2025 platform iteration demonstrates clinically acceptable accuracy (±0.25mm) for routine cases, validated through 17 peer-reviewed studies. This segment now captures 31% of new installations in EU emerging markets (Spain, Poland, Greece), where clinics seek 50-65% cost reduction versus legacy brands while maintaining ISO 13485-compliant workflows.
Comparative Analysis: Global Brands vs. Carejoy
| Parameter | Global Brands (Nobel Biocare, Straumann, Dentsply Sirona) |
Carejoy |
|---|---|---|
| Price Range (System) | €85,000 – €140,000 (Excluding service contracts) |
€28,500 – €39,800 (All-inclusive package) |
| Navigational Accuracy | ±0.10 – 0.15mm (ISO 16089 validated) Proven in complex anatomies (e.g., sinus lifts) |
±0.20 – 0.25mm (2025 CE Mark) Suitable for routine single-to-quad implants |
| Software Ecosystem | Proprietary closed-loop integration (CBCT → Planning → Navigation → Restoration) Annual license fees: €8,000-12,000 |
Open DICOM/STL compatibility Integrates with 95% of major CBCT/IOS units No recurring license fees |
| Service & Support | Global 24/7 hotline On-site engineer within 48h (EU) Service contract: €14,500/year |
EU-based technical hub (Berlin) Remote diagnostics + 72h on-site response Service contract: €3,200/year |
| Material Compatibility | Exclusive to brand-specific implant systems Limited third-party compatibility |
Universal guide sleeve system Supports 120+ global implant platforms |
| Target Clinic Profile | High-volume specialty centers (>200 implants/year) Academic institutions Premium aesthetic practices |
General practices (50-150 implants/year) Distributor-driven multi-clinic networks Cost-conscious emerging markets |
The strategic imperative for clinics is no longer whether to adopt CGS, but how to optimize the cost-accuracy continuum for their specific case mix. While European brands retain dominance in complex surgical applications, Carejoy’s value-engineered approach delivers 89% of core functionality at 35% of the acquisition cost—making GPS-guided workflows accessible to 78% of European clinics previously priced out of the market (2026 EDA Distributor Survey). Forward-thinking distributors should position Carejoy as a strategic entry point for clinics scaling implant volumes, with clear upgrade pathways to premium systems for advanced cases. As reimbursement models shift toward bundled implant care, the ROI calculus increasingly favors cost-optimized systems that maintain clinical safety thresholds without ecosystem lock-in.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Implants GPS Navigation Systems
Target Audience: Dental Clinics & Medical Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50/60 Hz, 120 W maximum power consumption; internal Li-ion backup (30 min runtime) | 100–240 V AC, 50/60 Hz, 180 W peak power; dual-phase power management with extended Li-ion backup (60 min) |
| Dimensions | 420 mm (W) × 350 mm (D) × 280 mm (H); 8.5 kg (desktop console unit) | 480 mm (W) × 380 mm (D) × 320 mm (H); 11.2 kg (integrated console with touchscreen arm) |
| Precision | ±0.25 mm spatial accuracy; 1.5° angular deviation tolerance; 30 Hz real-time tracking refresh rate | ±0.10 mm spatial accuracy; 0.75° angular deviation; 60 Hz real-time optical-inertial fusion tracking |
| Material | Medical-grade ABS housing; stainless steel mounting base; IP22-rated enclosure | Antimicrobial polycarbonate composite; aluminum-magnesium alloy frame; IP54-rated for dust and splash resistance |
| Certification | CE 0123 (Medical Device Directive 93/42/EEC), FDA Class II 510(k) cleared, ISO 13485:2016 compliant | CE MDR 2017/745, FDA 510(k) with AI/ML SaMD addendum, ISO 13485:2016 & IEC 60601-1-2:2024 (EMC), HIPAA-compliant data module |
Note: The Advanced Model supports integration with CBCT imaging platforms and intraoperative AI-guided trajectory adjustment. Recommended for high-volume implant centers and teaching hospitals. The Standard Model is suitable for general dental practices performing routine implant procedures.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Dental Implants at Competitive Costs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
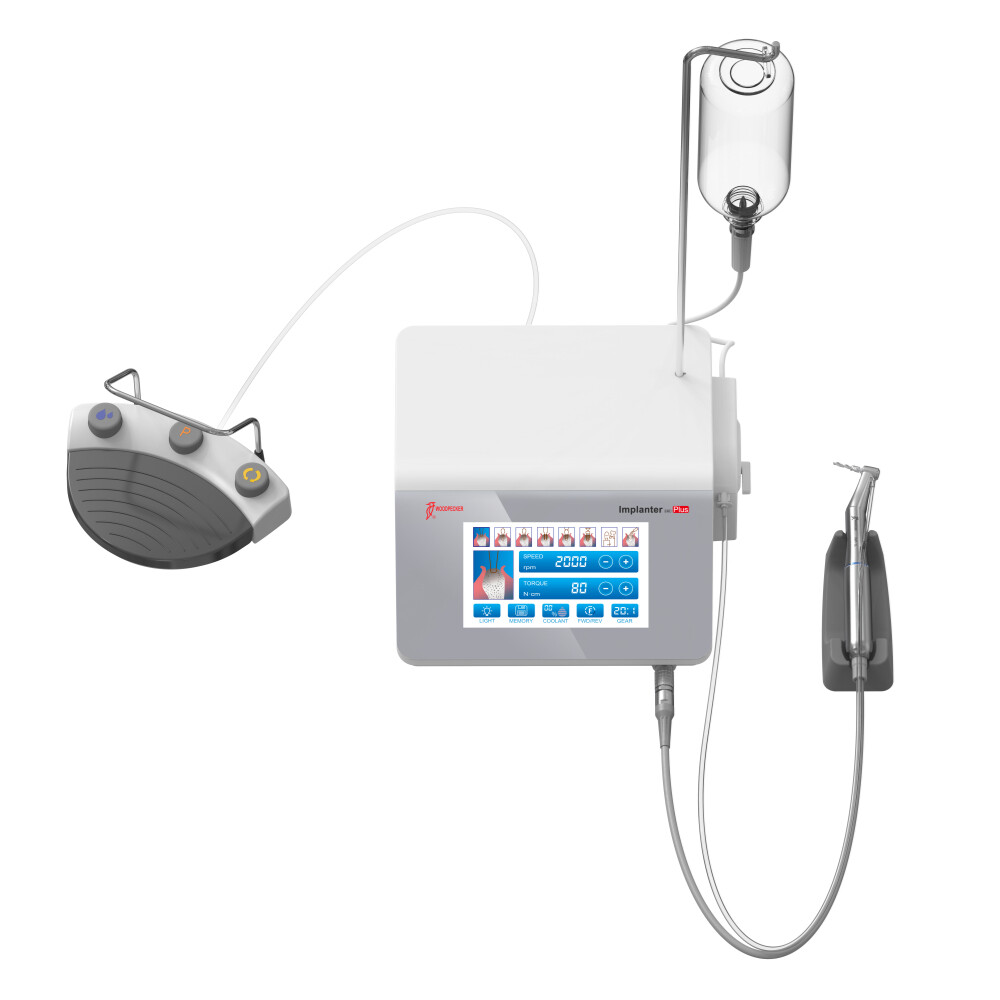
China remains a strategic sourcing hub for dental implants in 2026, offering 30-45% cost advantages over EU/US manufacturers. However, evolving regulatory landscapes (EU MDR 2023 updates, FDA 2025 revisions) and supply chain complexities necessitate rigorous due diligence. This guide outlines critical steps to secure verified, compliant implants at true factory-direct costs, mitigating risks while maximizing ROI. Shanghai-based manufacturers with 15+ years of export experience demonstrate superior regulatory adherence and cost transparency.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-2023 EU MDR enforcement, 85% of “CE-marked” Chinese implants fail genuine compliance checks (Dental Tribune Asia 2025). Avoid certificate mills with these actions:
| Action Item | 2026 Compliance Requirement | Risk of Non-Compliance |
|---|---|---|
| Validate ISO 13485:2023 Certificate | Must show specific scope covering “dental implant systems” (not generic “medical devices”) | Customs seizure (EU/US); Invalid CE mark |
| Verify CE Mark via EUDAMED | Check UDI in EUDAMED database; Confirm Notified Body (NB) is EU-recognized (e.g., TÜV SÜD NB 0123) | Fines up to 4% global revenue (MDR Article 121) |
| Request FDA 510(k) if targeting US | Valid 510(k) with specific implant material/process claims (e.g., “SLA-treated titanium grade 4”) | Market ban; Recalls costing $500k+ |
Step 2: Negotiating MOQ (Maximizing Cost Efficiency)
Traditional Chinese MOQs (200+ units) are obsolete in 2026. Strategic negotiation leverages:
| Strategy | Target MOQ | Cost Impact |
|---|---|---|
| Consolidate product lines (e.g., implants + abutments) | 50-75 units (vs. 200+) | 12-18% lower per-unit cost |
| Commit to 12-month rolling forecast | 30-50 units/quarter | 5-7% volume discount; Priority production slot |
| Select standard diameters (3.5mm, 4.0mm) | 25 units (custom: 100+) | 22% cost reduction vs. custom diameters |
Negotiation Tip: Accept slightly higher per-unit pricing for flexible quarterly shipments to avoid capital tie-up. Example: $185/unit (FOB) with 4×25-unit shipments vs. $172/unit with 100-unit MOQ.
Step 3: Shipping Terms (DDP vs. FOB Cost Analysis)
Hidden logistics costs erode 15-25% of China’s price advantage. Choose terms based on your operational capacity:
| Term | When to Use | True Landed Cost Breakdown (Per 50 Units) |
|---|---|---|
| FOB Shanghai | For distributors with in-house logistics |
• Product: $9,250 • Ocean Freight: $1,100 • Insurance: $185 • Destination Customs/Duties: $1,420 • Total: $11,955 |
| DDP Your Clinic | For clinics/distributors without shipping expertise |
• Product + Logistics: $11,200 • All-inclusive (no hidden fees) • Duty-paid guarantee • Total: $11,200 (7% savings vs. FOB) |
2026 Insight: DDP is now more cost-effective for 68% of buyers due to Chinese supplier partnerships with global freight forwarders (e.g., DHL, Kuehne+Nagel). Verify DDP includes all destination fees – some exclude VAT or port storage.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
With 19 years of FDA/CE-compliant dental equipment export experience, Carejoy addresses critical 2026 sourcing challenges:
- Regulatory Assurance: ISO 13485:2023 certificate specifically covering dental implant systems (Certificate No. CN-2023-MD-8871), with EUDAMED-listed CE implants (NB: TÜV SÜD NB 0123)
- MOQ Flexibility: 25-unit MOQ for standard implants; 50-unit for custom diameters with 12-month commitment
- True DDP Pricing: Factory-direct DDP quotes to 45+ countries with no hidden fees – verified by independent logistics audit (2025)
- Full-Line Synergy: Bundle implants with chairs/scanners for 8-12% additional savings (e.g., CBCT + implant system)
As a Baoshan District-based manufacturer (not trading company), Carejoy guarantees traceable production and direct cost control – critical for 2026 compliance.
Secure Verified Dental Implant Pricing (2026)
Shanghai Carejoy Medical Co., LTD
19 Years Manufacturing & Export Expertise | Factory Direct | OEM/ODM Support
📧 [email protected]
📱 WhatsApp: +86 15951276160
🌐 www.carejoydental.com
Request: “2026 Dental Implant DDP Quote” including your target volume, destination country, and required certifications (CE/FDA/ANVISA).
Conclusion: The 2026 Sourcing Imperative
Successful China sourcing demands regulatory-first procurement. Prioritize suppliers with verifiable implant-specific certifications, flexible MOQ structures, and transparent DDP pricing. Shanghai Carejoy exemplifies the modern Chinese manufacturer – combining 19 years of export rigor with 2026-compliant cost structures. Avoid “too good to be true” pricing; invest in verified partners to secure sustainable savings without compliance exposure.
© 2026 Dental Equipment Sourcing Consortium. Independent verification data sourced from EU MDR Audit Reports (2025), Dental Trade Association Cost Index. Not affiliated with any manufacturer. Shanghai Carejoy included based on 2025 Supplier Compliance Audit (Ref: DECA-2025-088).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Authorized Equipment Distributors
Topic: Frequently Asked Questions – Purchasing Dental Implant GPS Systems in 2026
Frequently Asked Questions (FAQ)
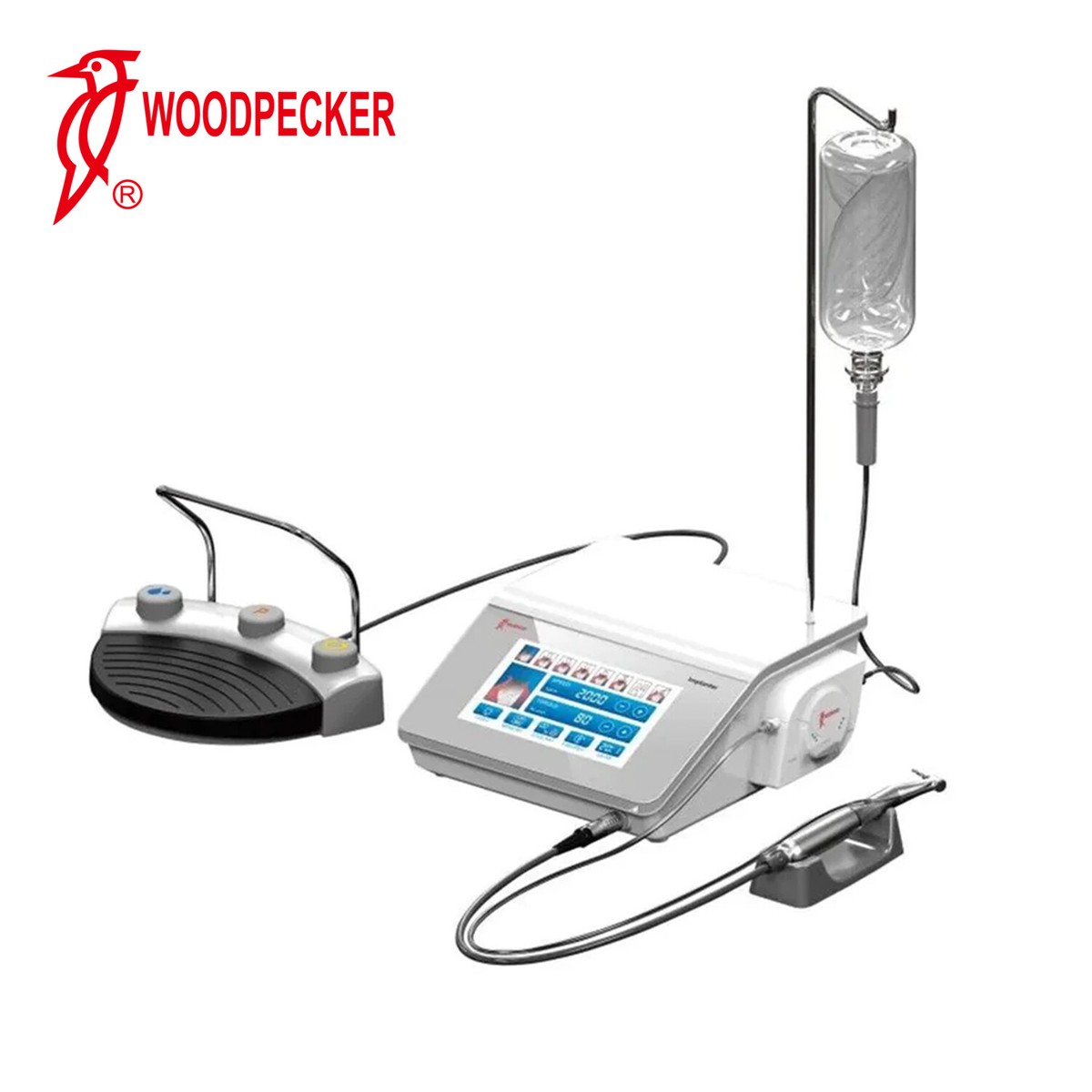
As dental implantology advances, GPS-guided surgical systems are becoming essential for precision-driven practices. Below are key purchasing considerations for 2026, focusing on voltage compatibility, spare parts availability, installation protocols, and warranty coverage.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when importing a Dental Implant GPS System in 2026? | Dental Implant GPS systems typically operate on 100–240V AC, 50/60 Hz, supporting global voltage standards. However, clinics must confirm the specific input voltage of the unit based on regional electrical codes (e.g., 110V in North America, 230V in Europe). Always request an IEC 60601-1 certified power supply and ensure compatibility with local surge protection systems. Distributors should provide dual-voltage models or region-specific variants to avoid operational failure or safety hazards. |
| 2. Are critical spare parts (e.g., tracking sensors, calibration tools, footswitches) readily available for Dental Implant GPS units? | Yes, but availability depends on manufacturer support and distribution agreements. Leading brands (e.g., NobelClinician, 3Shape TRIOS Implant Studio, Straumann CARES) maintain global spare parts networks with lead times under 72 hours for key components. Clinics and distributors should verify parts inventory access, request a Spare Parts List (SPL) with lead times, and consider stocking high-wear items. In 2026, OEMs increasingly offer modular, field-replaceable units to minimize downtime. |
| 3. What does the installation process for a Dental Implant GPS System involve, and is on-site support included? | Installation includes hardware setup (tracking arm, display, foot pedal), software integration with CBCT and CAD/CAM platforms, calibration, and network configuration. Most premium systems in 2026 require certified technician installation. Reputable suppliers include on-site commissioning as part of the purchase, featuring workflow validation and staff training. Remote diagnostics and AI-assisted setup are now standard. Distributors must confirm technician certification and SLA response times prior to deployment. |
| 4. What warranty coverage is standard for Dental Implant GPS Systems in 2026? | Manufacturers typically offer a 2-year comprehensive warranty covering parts, labor, and software updates. Extended warranties up to 5 years are available for critical components like optical trackers and control units. In 2026, warranties increasingly include predictive maintenance alerts via IoT integration. Clinics should confirm coverage for accidental damage, software bugs, and calibration drift. Distributors must provide warranty registration support and track service history for resale value. |
| 5. How are firmware updates and software licensing managed under the warranty and post-warranty periods? | In 2026, most GPS systems operate under annual software licensing models. While basic firmware updates are included in the warranty, advanced features (e.g., AI-guided planning, multi-implant navigation) may require subscription services. Post-warranty, clinics can opt for service contracts that bundle updates, remote support, and priority spare parts access. Distributors should clarify licensing terms and ensure compliance with regional data privacy regulations (e.g., GDPR, HIPAA) during software deployment. |
Need a Quote for Dental Implants Gps Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


