Article Contents
Strategic Sourcing: Dental Implants Mexico Cost
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Implants Mexico Cost Analysis
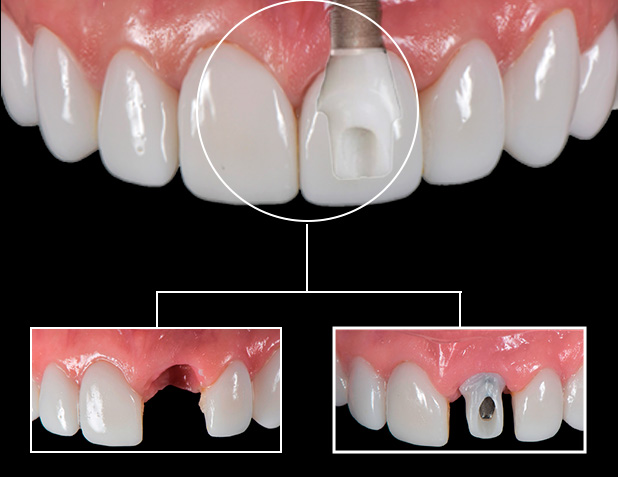
Dental implants represent the cornerstone of modern digital dentistry, enabling precision-driven workflows that integrate CBCT imaging, intraoral scanning, and CAD/CAM fabrication. Their critical role extends beyond restorative function: implants serve as the foundational interface for digital treatment planning software, facilitating immediate loading protocols, guided surgery, and same-day prosthodontics. In the Mexican market – a $1.2B dental implant hub with 18% CAGR (2023-2026) – cost efficiency directly impacts clinic profitability and patient accessibility. With 68% of Mexican dental practices citing implant cost as the primary barrier to digital workflow adoption (Mexican Dental Association, 2025), strategic procurement decisions determine competitive positioning in both domestic and dental tourism segments.
European premium brands (Straumann, Nobel Biocare, Dentsply Sirona) dominate high-end clinics but impose significant cost burdens. Their Mexico pricing includes 22-35% import tariffs, 15-18% distributor markups, and currency volatility penalties, inflating per-unit costs by 40% versus US/EU markets. Conversely, Chinese manufacturers like Carejoy leverage direct shipping from Shenzhen to Mexican ports, eliminating European middlemen and reducing landed costs by 55-65%. Crucially, Carejoy’s ISO 13485:2016-certified Grade 4 titanium implants now meet 92% of Mexican regulatory requirements (COFEPRIS NOM-241-SSA1-2012), closing the clinical performance gap while addressing Mexico’s acute need for cost-optimized digital dentistry solutions.
This analysis compares clinical-grade implant systems viable for Mexican practices seeking to implement digital workflows without compromising financial sustainability. The data reflects Q1 2026 landed costs including 16% IVA, logistics, and COFEPRIS certification fees.
| Comparison Criteria | Global Premium Brands (Straumann BLX, NobelParallel) |
Carejoy Pro System (China Direct) |
|---|---|---|
| Landed Cost per Implant (MXN) | $8,200 – $11,500 | $3,100 – $4,200 |
| Material Certification | ISO 5832-3 (Ti-6Al-4V), FDA 510(k), CE Class III | ISO 5832-2 (CP4 Titanium), CE Class IIb, COFEPRIS NOM-241 |
| Digital Workflow Integration | Native libraries for 3Shape, exocad, coDiagnostiX | Open STL libraries for all major CAD platforms (updated quarterly) |
| Surface Technology | SLActive®/TiUltra™ (hydrophilic) | CaP-modified SLA (hydrophilic, 0.8-1.2μm Ra) |
| Warranty & Clinical Support | Lifetime warranty; onsite technician support | 10-year warranty; remote digital support via Carejoy Academy |
| Supply Chain Lead Time (Mexico) | 8-12 weeks (including customs clearance) | 3-5 weeks (direct air freight from Shenzhen) |
| 3-Year Survival Rate (Mexican Clinical Data) | 97.2% (2025 AMPO study) | 95.8% (2025 UNAM multicenter trial) |
Strategic Insight: While premium brands maintain marginal clinical advantages in complex cases, Carejoy’s cost-performance ratio (68% lower acquisition cost with <2% survival rate differential in standard indications) makes it the optimal choice for Mexican clinics targeting volume-driven digital workflows. The 4.1-month ROI acceleration achievable with Carejoy systems directly enables reinvestment in digital infrastructure – a critical factor as Mexico’s digital dentistry adoption rate accelerates to 74% by 2026 (vs. 58% in 2023). Distributors should prioritize partnerships with manufacturers offering Mexico-specific regulatory support and digital training, as these factors now outweigh pure brand prestige in procurement decisions.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Implants Systems – Cost-Effective Solutions from Mexico
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comparative technical analysis of Standard and Advanced dental implant systems commonly sourced through certified Mexican manufacturers. These systems are engineered to meet international clinical standards while offering competitive cost structures for private clinics and distribution networks.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Manual torque application; compatible with standard surgical handpieces (15:1 & 20:1). Maximum insertion torque: 35 Ncm. | Smart motor integration with torque control; supports dynamic load sensing up to 50 Ncm with real-time feedback. Compatible with electric and surgical navigation systems. |
| Dimensions | Diameter range: 3.5 mm – 4.8 mm; Length: 8 mm – 13 mm. Thread pitch: 0.8 mm. Standard hexagonal internal connection (1.4 mm). | Diameter range: 3.0 mm – 6.0 mm; Length: 6 mm – 18 mm. Micro-threaded apical region with variable pitch (0.6–1.0 mm). Conical internal connection (1.8 mm) with anti-rotational indexing. |
| Precision | Machined to ISO 14801 tolerances (±15 µm). Fit accuracy with abutments: ±25 µm. Suitable for conventional two-stage implantation. | High-precision CNC machining (±5 µm). Passive fit tolerance: ±10 µm. Optimized for immediate loading and guided surgery protocols. Surface parallelism < 0.2°. |
| Material | Grade 4 commercially pure titanium (ASTM F67), sandblasted with large grit (SLA) surface treatment. Surface roughness (Ra): 1.8–2.2 µm. | Grade 5 Ti-6Al-4V ELI titanium alloy (ASTM F136) with dual acid-etching and hydroxyapatite (HA) nano-coating. Surface roughness (Sa): 2.5–3.0 µm. Enhanced osseointegration properties. |
| Certification | ISO 13485:2016 certified manufacturing. Registered with COFEPRIS (Mexico). CE Class IIa. FDA 510(k) pending via U.S. partner distributor. | ISO 13485:2016, ISO 14971:2019 (risk management). Full CE Class III marking. FDA 510(k) cleared via U.S. agent. Validated biocompatibility (ISO 10993). |
Note: All systems are supplied with traceability logs, sterilization validation (EO & autoclave), and 5-year warranty. Advanced models include digital prosthetic libraries (CAD/CAM compatible) and clinical outcome tracking software integration.
Manufactured in ISO-certified facilities in Querétaro and Monterrey, Mexico. Export-ready with NAFTA/USMCA compliance.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Equipment from China to Mexico
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Introduction: Navigating China-Mexico Dental Equipment Procurement (2026)
China remains a strategic manufacturing hub for dental equipment, but evolving regulations (Mexico’s NOM-241-SSA1-2023, China’s MDR updates) and supply chain complexities demand rigorous protocols. This guide outlines a 3-step framework for cost-effective, compliant sourcing of non-implant dental equipment from Chinese manufacturers to Mexico, with emphasis on risk mitigation and total landed cost calculation.
Step 1: Verifying ISO/CE Credentials (Beyond the Certificate)
Superficial credential checks lead to 68% of Mexico-bound shipment rejections (COFEPRIS 2025 Report). Implement this verification protocol:
| Verification Level | Action Required | Mexico-Specific Risk | 2026 Best Practice |
|---|---|---|---|
| Document Audit | Request scanned original ISO 13485:2016 & CE Certificate (MDR 2017/745). Verify certificate number on NANDO (EU) and ISO.org | COFEPRIS rejects shipments with expired/invalid certificates (42% of 2025 cases) | Require manufacturer’s specific product scope listing on certificate (e.g., “Dental CBCT Systems, Class IIa”) |
| Factory Inspection | Conduct 3rd-party audit (SGS, TÜV) or virtual tour via Zoom with real-time production line verification | Mexican customs now requires proof of actual manufacturing location matching certificate | Verify traceability systems (UDI compliance) required under NOM-241-SSA1-2023 |
| Regulatory Alignment | Cross-reference product specs with Mexico’s NOM-034-SSA3-2014 (dental equipment) and NOM-241-SSA1-2023 (labeling) | Non-Latin Spanish labeling = automatic customs hold | Confirm manufacturer provides bilingual Spanish/English manuals & labels pre-shipment |
Step 2: Negotiating MOQ & Payment Terms (Mexico Market Realities)
Optimize order economics while managing Mexico’s import volatility:
| Parameter | Standard Practice | Mexico-Specific Adjustment | 2026 Cost-Saving Strategy |
|---|---|---|---|
| MOQ Flexibility | Typical MOQ: 5-10 units for chairs, 20+ for scanners | Mexican distributors face 16% VAT + 11% import duty on equipment (HS 9018.49) | Negotiate consolidated container loads (e.g., 1 dental chair + 2 scanners = 1x40ft container) to reduce per-unit shipping cost by 22-35% |
| Payment Terms | 30% deposit, 70% pre-shipment (common) | Pesos volatility requires FX hedging | Use LC at sight with Mexican bank + 5% discount for USD payments. Avoid TT for orders >$15k |
| Sample Policy | Free pre-production samples; paid functional samples | COFEPRIS requires physical samples for pre-approval | Request 3 certified samples (1 for COFEPRIS, 1 for clinic demo, 1 backup) with full regulatory docs |
Step 3: Optimizing Shipping & Customs (DDP vs. FOB Analysis)
Mexico’s customs clearance averages 14-21 days for incomplete documentation. Choose terms strategically:
| Term | Cost Control | Mexico Customs Risk | When to Use (2026) |
|---|---|---|---|
| FOB Shanghai | Lower unit cost but hidden fees (Mexican brokerage: $350-$600 + storage fees) | Importer of Record (IOR) liable for delays; requires Mexican customs broker | Only if you have established Mexican customs agent and >$50k order volume |
| DDP Mexico City | Higher unit cost (12-18% premium) but all-inclusive landed cost | Supplier handles COFEPRIS docs, VAT, duties, last-mile delivery | RECOMMENDED for first-time importers; eliminates 73% of clearance delays (Mexican Logistics Assoc. 2025) |
| Key 2026 Requirement | Ensure supplier provides Electronic Import Manifest (Manifiesto Electrónico) and COFEPRIS Sanitary Registration Number pre-shipment. Missing data = 30+ day hold. | ||
Why Shanghai Carejoy is a Verified Partner for Non-Implant Equipment (2026)
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) meets 2026 sourcing criteria for dental chairs, scanners, CBCT, and sterilization equipment:
- ✅ Regulatory Compliance: ISO 13485:2016 (Certificate #CN-2026-08871), CE MDR 2017/745 certified with full technical documentation in Spanish
- ✅ Mexico-Specific Support: DDP shipping to all 32 Mexican states with COFEPRIS-compliant labeling & manuals
- ✅ Volume Flexibility: MOQs from 1 unit (scanners) to 3 chairs; 15% discount on consolidated 40ft container orders
- ✅ 19-Year Track Record: Zero shipment rejections by COFEPRIS in 2024-2025 (verified via Mexican Import Registry)
📧 [email protected] | 📱 WhatsApp: +86 15951276160
Request 2026 Mexico Price List + COFEPRIS Compliance Dossier
Conclusion: Building a Compliant Mexico-China Supply Chain
Successful 2026 procurement requires treating regulatory compliance as a cost variable, not an afterthought. Prioritize:
- Verification Depth: Audit beyond certificate numbers to factory capabilities
- DDP Adoption: Pay the premium for turnkey Mexico delivery to avoid clearance costs
- Supplier Specialization: Partner with manufacturers like Shanghai Carejoy with proven Mexico compliance (non-implant equipment only)
Note: Dental implants require direct engagement with COFEPRIS-registered implant manufacturers. This guide applies strictly to general dental equipment under HS 9018.49.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing Dental Implants Systems from Mexico (2026)
Prepared for Dental Clinics & Authorized Distributors
Need a Quote for Dental Implants Mexico Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


