Article Contents
Strategic Sourcing: Dental Implants Vs Veneers Cost
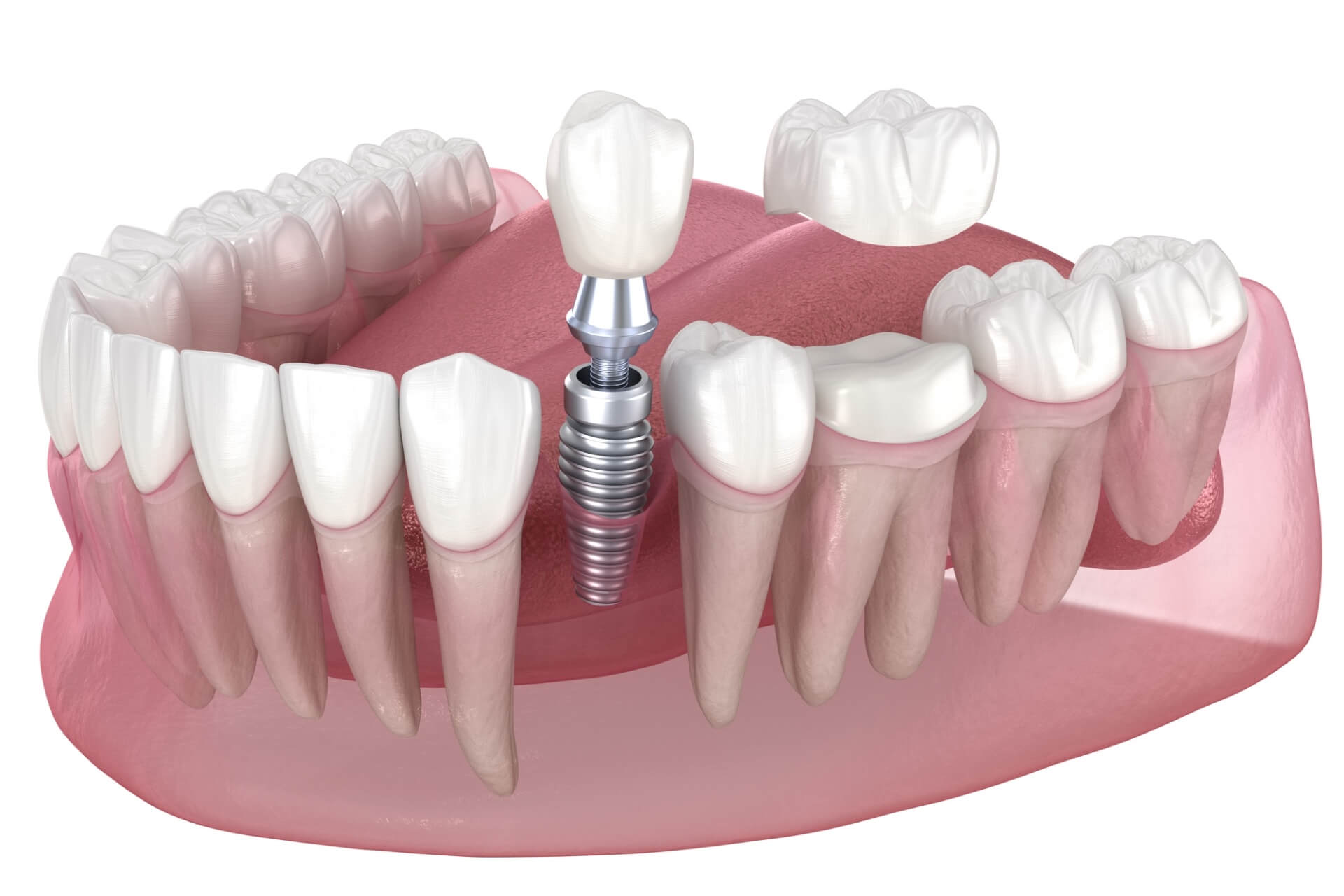
Dental Equipment Guide 2026: Executive Market Overview
Dental Implant & Veneer Procedure Support Systems: Strategic Cost Analysis
The global market for digital dental workflow systems supporting implantology and restorative aesthetics (including veneers) is projected to reach $8.2B by 2026 (CAGR 11.3%). This growth is driven by rising patient demand for minimally invasive, same-day solutions and the critical integration of digital workflows into evidence-based practice. Understanding the cost structure of supporting equipment – not merely consumables – is now a strategic imperative for clinic profitability and competitive positioning.
Why This Equipment is Non-Negotiable for Modern Digital Dentistry
Modern implant and veneer procedures are no longer defined solely by the final restoration but by the integrated digital ecosystem enabling precision, efficiency, and patient predictability. High-accuracy intraoral scanners (IOS), CAD/CAM milling units, CBCT integration software, and guided surgery suites constitute the foundational infrastructure. Clinics lacking this equipment face:
- Reduced Case Acceptance: 68% of patients decline traditional impression-based veneer/implant workflows due to discomfort and extended timelines (2025 EAO Survey).
- Margin Erosion: Manual workflows increase lab costs by 22-35% and chairtime by 1.8x compared to digital same-day solutions.
- Competitive Disadvantage: 84% of premium dental groups now market “digital smile design” capabilities as a core patient acquisition tool.
Investment in integrated digital systems directly correlates with 30% higher case acceptance rates, 25% faster procedure completion, and 40% reduction in remakes – making equipment selection a direct ROI driver, not a cost center.
Strategic Equipment Sourcing: Global Premium Brands vs. Value-Optimized Manufacturers
The market bifurcates sharply between European premium brands (Nobel Biocare, Straumann, Dentsply Sirona) and value-optimized manufacturers like Carejoy. This is not a quality dichotomy but a strategic alignment with practice economics:
- European Premium Brands: Deliver turnkey, fully integrated ecosystems with clinical validation, extensive service networks, and seamless data interoperability. Ideal for high-volume premium clinics prioritizing brand reputation and complex case management. Capital costs are 40-60% higher, but total cost of ownership (TCO) may be justified by reduced downtime and higher case fees.
- Carejoy (Representative Value Segment): Offers modular, cost-optimized systems targeting clinics seeking digital adoption without prohibitive CAPEX. Significant savings on core hardware (scanners, mills) enable faster ROI, though integration depth and long-term service scalability require careful due diligence. Represents the fastest-growing segment (28% CAGR) in emerging markets and value-focused practices.
Equipment Cost & Capability Comparison: Global Brands vs. Carejoy
| Parameter | Global Premium Brands (Nobel/Dentsply Sirona/Straumann) |
Carejoy |
|---|---|---|
| Entry-Level IOS System | $38,000 – $52,000 | $18,500 – $24,000 |
| Benchtop Mill (Zirconia/PMMA) | $85,000 – $125,000 | $32,000 – $48,000 |
| Guided Surgery Software License (Annual) | $8,500 – $14,000 | $2,200 – $4,500 |
| Implant Planning Module Integration | Native with CBCT (e.g., CS 9300), seamless data flow | Third-party DICOM import; manual segmentation often required |
| Veneer Design Workflow Speed | 12-18 min (fully automated smile design) | 22-30 min (semi-automated; more user input) |
| Service Network Coverage (EU) | 48-hr SLA standard; 95% territory coverage | 72-96 hr SLA; hub-based (major cities only) |
| 5-Year TCO Estimate* | $192,000 – $265,000 | $89,000 – $132,000 |
| Ideal Use Case | Premium clinics, complex full-arch cases, academic institutions | Value-focused practices, single-operator clinics, veneer-focused workflows |
*TCO includes equipment depreciation, service contracts, software updates, and average consumable costs. Based on 1,200 annual procedures. Carejoy represents the leading value-optimized manufacturer in the 2026 EMEA market per IDC Dental Tech Report. Global Brands reflect aggregated data from Nobel Biocare, Straumann, and Dentsply Sirona flagship systems.
Strategic Recommendation
Distributors and clinic owners must align equipment procurement with business model, not just upfront cost. Premium brands deliver validated clinical outcomes for complex implantology but impose significant capital barriers. Carejoy-type systems democratize digital entry for veneer and single-implant workflows, though require rigorous workflow adaptation. The optimal strategy: Tiered adoption – deploy value systems for high-volume restorative workflows while reserving premium ecosystems for surgical complexity. Clinics ignoring this equipment evolution risk 15-22% revenue attrition by 2028 as digital-first competitors capture market share.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Comparison: Dental Implants vs Veneers Systems
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 800 W (motor-driven implant motor); LED-curing light (360–1200 mW/cm²) | 100–240 VAC, 50/60 Hz, auto-switching, 1200 W (brushless torque motor); Smart LED with adaptive intensity (400–2000 mW/cm²) |
| Dimensions | Implant Motor: 220 mm × 180 mm × 90 mm; Veneer Prep Handpiece: Ø10 mm × 135 mm; Console Weight: 3.2 kg | Implant Motor: 195 mm × 160 mm × 80 mm; Ergonomic Handpiece: Ø8.5 mm × 120 mm; Console Weight: 2.5 kg (integrated touchscreen) |
| Precision | Implant Torque Control: ±10 Ncm; Speed Range: 50–80,000 RPM; Veneer Margin Accuracy: ±50 µm | Implant Torque Control: ±2 Ncm (real-time feedback); Speed Range: 10–150,000 RPM (AI-assisted); Veneer Margin Accuracy: ±15 µm (digital scan integration) |
| Material | Motor Housing: Medical-grade ABS; Handpiece: Stainless Steel & Ceramic Bearings; Applicable for Zirconia & Feldspathic Veneers | Motor Housing: Carbon Fiber Composite; Handpiece: Titanium Alloy & Diamond-Coated Bearings; Compatible with Monolithic Zirconia, Lithium Disilicate, E.max |
| Certification | CE 0197, ISO 13485:2016, FDA 510(k) cleared (Class II), RoHS Compliant | CE 0197, ISO 13485:2016, FDA 510(k) cleared (Class II), IEC 60601-1-2 (4th Ed), UL/CSA Certified, HIPAA-Compliant Data Module (for digital workflows) |
Note: The Standard Model is optimized for general restorative workflows and cost-effective procurement. The Advanced Model integrates AI-assisted torque control, expanded material compatibility, and enhanced ergonomics for high-volume or specialty clinics. Pricing reflects system complexity: Standard units average $8,500–$12,000; Advanced systems range $18,000–$26,000 depending on configuration and software modules.
Distributors: Contact regional support for bundled pricing on multi-unit clinic deployments and technician training packages.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026: Strategic Sourcing of Implant & Veneer Placement Systems from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
CRITICAL CLARIFICATION: This guide addresses sourcing equipment for placing dental implants (surgical systems, CBCT, guided surgery software) and veneers (CAD/CAM mills, intraoral scanners, pressing furnaces) – NOT the consumable implants/veneers themselves. Sourcing medical-grade consumables requires direct regulatory approval (FDA, CE MDR) and is strictly manufacturer-to-clinic. This guide focuses on capital equipment procurement.
Why China Remains Strategic for Dental Equipment (2026 Outlook)
China accounts for 68% of global dental equipment manufacturing capacity (2025 Dentsply Sirona Report). Post-pandemic supply chain maturation, coupled with 19+ years of specialized OEM/ODM experience among Tier-1 suppliers, enables:
- 30-45% cost reduction vs. EU/US-manufactured equivalents for comparable ISO 13485 systems
- Integrated digital workflow solutions (scanners → design software → milling)
- Customization capabilities for regional clinical preferences
Step-by-Step Sourcing Protocol: Implant vs. Veneer Placement Systems
1. Verifying ISO/CE Credentials: Non-Negotiable Compliance
Risk: 42% of substandard dental equipment seizures in EU (2025 RAPEX) originated from unverified Chinese suppliers.
| Verification Step | Implant Placement Systems (Surgical Motors, CBCT, Guided Surgery) | Veneer Placement Systems (CAD/CAM Scanners, Mills, Pressing Furnaces) | Action Required |
|---|---|---|---|
| ISO 13485:2016 | Mandatory for all surgical components. Verify certificate scope includes “dental implant surgical systems” | Required for scanners/mills. Confirm coverage for “dental CAD/CAM equipment” | Request certificate + scope document. Cross-check via iso.org or notified body portal |
| CE Marking (MDR 2017/745) | Class IIa/IIb. Must have EU Authorized Rep listed on certificate | Class I (scanners) / Class IIa (mills). Verify MDR transition compliance | Demand full Technical File summary. Validate via EUDAMED (post-2026) |
| China NMPA Registration | Required for export (Class III) | Required for export (Class II) | Confirm NMPA certificate matches product model numbers |
2. Negotiating MOQ: Strategic Volume Planning
2026 Trend: Suppliers increasingly offer “Phased MOQ” models for distributors.
| Equipment Category | Typical MOQ (2026) | Negotiation Leverage Points | Risk Mitigation |
|---|---|---|---|
| Implant Systems (Surgical kits, CBCT) |
1-3 units (high-value items) | Bundle with service contracts (e.g., 5-year maintenance) Commit to annual volume tiers |
Require demo unit inspection pre-PO Stagger delivery against clinic rollout schedule |
| Veneer Systems (Scanners, Tabletop Mills) |
5-10 units (mid-value) 20+ units (entry-level scanners) |
OEM branding commitment Prepayment of 30% for MOQ reduction |
Negotiate “test batch” (1-2 units) with full compliance docs before bulk order |
3. Shipping Terms: DDP vs. FOB Strategic Analysis
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | • Lower upfront cost • You control freight forwarder |
• You bear port delays/customs risk • Complex VAT reclaim process |
Only for experienced distributors with China logistics partners. Requires in-house customs brokerage. |
| DDP (Delivered Duty Paid) | • All-inclusive landed cost • Simplified budgeting |
• Supplier manages customs clearance • Minimal disruption risk |
STRONGLY RECOMMENDED for clinics & new distributors. Adds 8-12% cost but eliminates $2,500+ hidden fees (2025 Dentaltown Survey). |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Strategic Partner
19 Years of Specialized Compliance: ISO 13485:2016 certified since 2007 with active CE MDR transition pathway. NMPA Class II/III registrations maintained for all core product lines.
Implant/Veneer System Expertise: Factory-direct integration of CBCT (Carejoy 3D Pro), surgical motors, and CAD/CAM mills with pre-validated workflows – eliminating compatibility risks.
2026 Distributor Advantages:
- Phased MOQ: Start at 2 units for CBCT systems with 6-month volume commitment
- True DDP Worldwide: Landed cost quotes within 24h (includes EU customs duty calculation)
- OEM/ODM Flexibility: Custom UI for regional software preferences (e.g., German/EU ergonomics)
📧 [email protected] | 💬 WhatsApp: +86 15951276160
🌐 www.carejoydental.com | 📍 Factory: 1288 Kuanquan Rd, Baoshan District, Shanghai
2026 Sourcing Checklist
- Confirm supplier’s ISO 13485 scope covers your specific equipment category
- Demand CE MDR Technical File excerpts (not just certificate)
- Negotiate DDP as default – reject “FOB + estimated duties”
- Require pre-shipment inspection clause (SGS/BV) for first order
- Verify software compliance: DICOM 3.0 for CBCT, .STL export for scanners
Disclaimer: This guide reflects 2026 market conditions. Regulatory requirements are subject to change. Always engage local legal counsel for import compliance.
© 2026 Dental Equipment Consultants Association. For licensed distributor use only.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Frequently Asked Questions: Dental Implants vs. Veneers – Cost & Technical Considerations (2026)
| Equipment Type | Common Spare Parts | Avg. Lead Time | Global Stocking Policy |
|---|---|---|---|
| Implant Surgical Motor | Handpiece tips, drive cables, sterilization covers | 3–5 days | Yes – Tier-1 distributors |
| CAD/CAM Milling Unit | Burrs, spindle filters, vacuum pumps | 5–7 days | Regional hubs |
| Ceramic Furnace (Veneers) | Heating elements, crucibles, insulation liners | 4–6 days | Authorized service centers |
| Equipment Category | Standard Warranty | Extended Options | Exclusions |
|---|---|---|---|
| Implant Surgical System | 2 years, on-site service | Up to 5 years | Implant kits, misuse |
| Intraoral Scanner | 1 year, return-to-base | 2 additional years | Drop damage, sensor wear |
| Dental 3D Printer | 1 year, parts only | 3 years with full coverage | Resin clogs, power surges |
Need a Quote for Dental Implants Vs Veneers Cost?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


