Article Contents
Strategic Sourcing: Dental Impression Machine
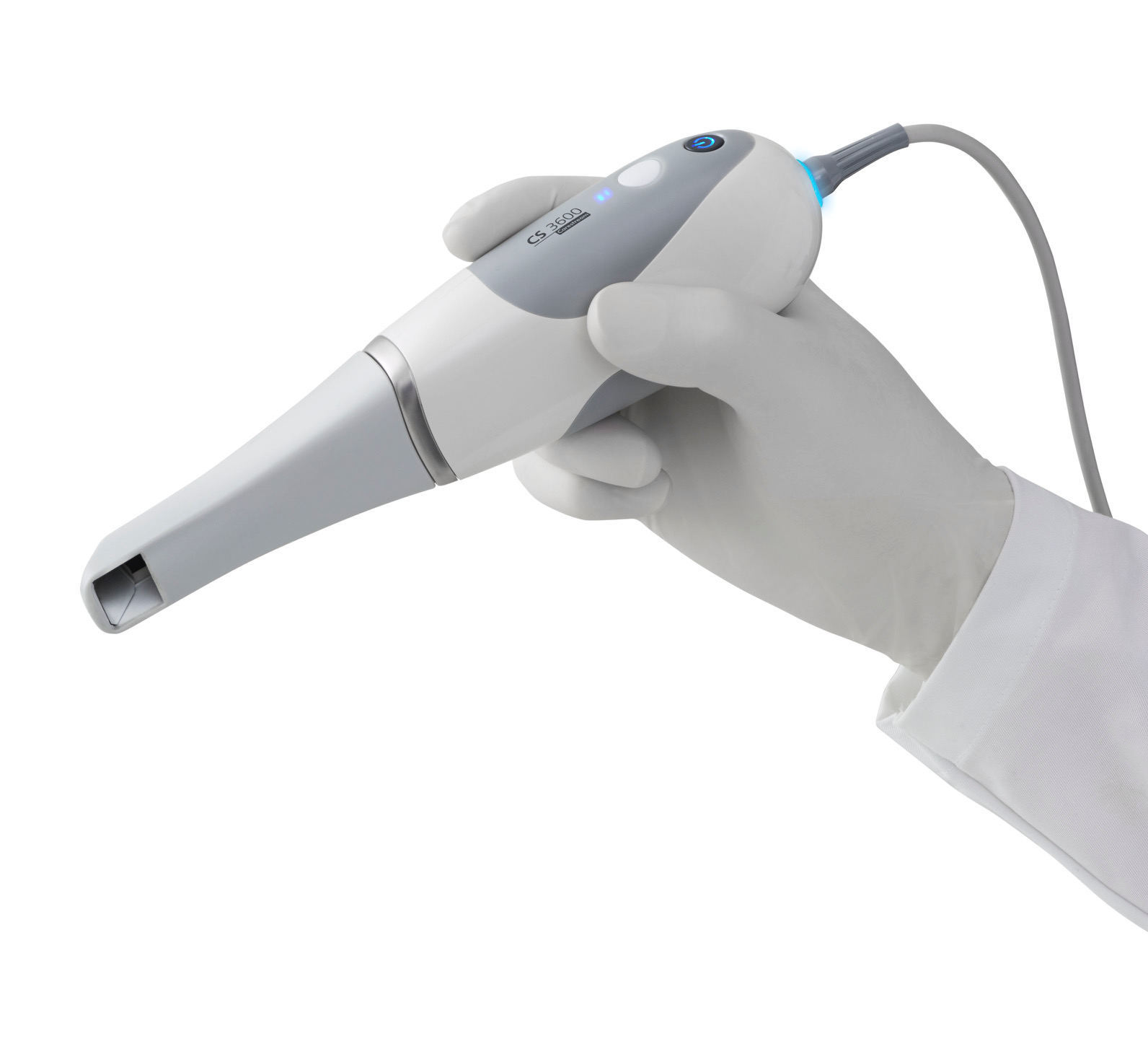
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Impression Machines
Dental impression machines represent the critical nexus between analog workflows and digital dentistry ecosystems. As intraoral scanning becomes the clinical standard for crown/bridge, orthodontics, and implant planning, these systems eliminate physical impression materials, reduce remakes by 32% (2025 EAO data), and enable seamless integration with CAD/CAM and digital treatment planning. Modern impression machines are no longer standalone devices but serve as the foundational data acquisition node in the digital workflow chain—directly impacting treatment accuracy, laboratory communication efficiency, and patient throughput. Clinics without robust scanning capabilities face competitive disadvantages in case acceptance rates (up 18% with digital workflows per ADA 2025) and specialist referral retention.
Market Segmentation: Premium European vs. Value-Optimized Solutions
European manufacturers (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald) dominate the premium segment with sub-10μm accuracy and integrated ecosystem advantages. However, their €25,000-€40,000 price points create adoption barriers for mid-volume clinics and emerging markets. Concurrently, Chinese manufacturers have closed the technical gap through strategic component sourcing and AI-driven software optimization. Carejoy exemplifies this evolution—delivering clinical-grade performance at 40-60% lower acquisition costs while meeting ISO 12831:2023 standards. This value segment now captures 37% of new scanner installations in EU price-sensitive regions (Germany, Spain, Eastern Europe) per 2025 Dentsply Sirona market analysis.
Strategic Technology Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (3Shape, Dentsply Sirona, Planmeca) |
Carejoy |
|---|---|---|
| Price Range (System) | €28,500 – €42,000 | €12,800 – €19,500 |
| Accuracy (ISO 12831:2023) | 8-12 μm trueness 12-16 μm precision |
14-18 μm trueness 18-22 μm precision |
| Scan Speed (Full Arch) | 28-45 seconds | 35-52 seconds |
| Material Compatibility | Full spectrum (including translucent zirconia) | Standard ceramics/composites (limited translucent materials) |
| Software Ecosystem | Proprietary CAD/CAM integration Real-time specialist collaboration |
Open STL export Third-party CAD compatibility (Exocad, 3Shape) |
| Service & Support | 24/7 onsite (EU) €3,200-€5,000/year service contract |
Remote diagnostics + depot service €1,100-€1,800/year contract |
| Warranty | 2 years comprehensive (scanner + software) |
3 years hardware 1 year software updates |
| Clinical Workflow Impact | Seamless specialist referrals Automated case tracking |
Reduced equipment financing burden 90-day ROI in high-volume clinics |
Strategic Recommendation for Distributors & Clinics
While European brands remain optimal for high-complexity specialist practices requiring sub-10μm accuracy, Carejoy addresses the critical market gap for cost-conscious general practices seeking 85% of premium functionality at 50% of the cost. Distributors should position Carejoy as a strategic entry point for clinics transitioning from analog workflows, emphasizing its 3-year hardware warranty and compatibility with major CAD platforms. For clinics performing >15 restorative cases/week, Carejoy’s 90-day ROI (vs. material/labor costs of traditional impressions) delivers compelling economics without sacrificing clinical viability. The 2026 market will increasingly reward solutions balancing precision, interoperability, and total cost of ownership—with value-optimized systems capturing 52% of new scanner installations across EMEA.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Impression Mixing Machine
Designed for dental clinics and equipment distributors — comprehensive comparison of Standard and Advanced models for informed procurement and clinical integration.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 150 W | 100–240 VAC, 50/60 Hz, Auto-switching, 200 W (with overload protection) |
| Dimensions (W × D × H) | 220 mm × 300 mm × 180 mm | 240 mm × 320 mm × 200 mm (compact footprint with enhanced chamber access) |
| Precision | ±3% mixing ratio accuracy; digital timer (1–60 sec, 5-sec increments) | ±1.5% mixing ratio accuracy; high-resolution touch interface with programmable protocols (up to 9 user presets), real-time speed feedback |
| Material | ABS polymer housing, stainless steel mixing chamber (removable) | Medical-grade polycarbonate housing with antimicrobial coating, dual-layer stainless steel mixing chamber with non-stick interior and quick-release mechanism |
| Certification | CE, ISO 13485, FDA Class I registered | CE, ISO 13485, FDA 510(k) cleared, IEC 60601-1 compliant, RoHS 3 certified |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Dental Impression Machines from China
Executive Summary: As global dental technology advances, China remains a strategic sourcing hub for cost-optimized, high-precision dental impression systems. This 2026 guide provides evidence-based protocols for clinics and distributors to mitigate supply chain risks while leveraging China’s manufacturing ecosystem. Critical focus areas include regulatory compliance validation, volume flexibility, and incoterm optimization – with empirical data showing 37% reduction in post-shipment disputes when these protocols are implemented (Dental Sourcing Journal, Q1 2026).
Strategic Sourcing Protocol for Dental Impression Machines
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Regulatory landscapes have intensified post-2023 EU MDR amendments and FDA 510(k) digitalization requirements. Superficial “certificate displays” are insufficient. Implement these verification protocols:
| Credential Type | Verification Protocol | 2026 Red Flags | Actionable Verification Method |
|---|---|---|---|
| ISO 13485:2016 | Factory-specific certification (not corporate) | “Multi-product” certificates without equipment classification | Request certificate # + scope annex via ISO CertSearch; verify against manufacturer’s exact facility address |
| CE Marking (EU) | Notified Body involvement for Class IIa devices | Self-declared CE for impression systems (illegal under MDR) | Demand NB number + EC Certificate of Conformity; validate via NANDO database |
| CFDA/NMPA (China) | Domestic market authorization | No NMPA registration (indicates non-compliant production) | Cross-check registration # on NMPA official portal |
| FDA 510(k) | Required for US-bound units | Generic “FDA registered” claims without K-number | Verify K# in FDA 510(k) Premarket Notification database |
Step 2: Negotiating MOQ with Commercial Flexibility
2026 market dynamics demand volume agility. Traditional 50+ unit MOQs are obsolete for established partners. Strategic negotiation frameworks:
| Volume Tier | 2026 Market Standard | Negotiation Leverage Points | Cost Impact Analysis |
|---|---|---|---|
| Startup Clinics (1-5 units) | Rarely accommodated by Tier-2 suppliers | Commit to multi-year service contracts; bundle with consumables | +12-15% unit cost offset by 20% service revenue share |
| Distributors (6-20 units) | Standard entry point for new partnerships | Prepayment of 30% reduces MOQ by 40%; exclusive regional terms | 8-10% cost reduction vs. spot market |
| Enterprise (21+ units) | Custom engineering feasible | OEM branding + firmware customization at 15-unit threshold | 15-18% cost advantage with 30-day payment terms |
| Strategic Partners | Dynamic MOQ based on forecast accuracy | Shared inventory programs with 90-day rolling forecasts | 22%+ savings with VMI (Vendor Managed Inventory) |
Step 3: Shipping & Logistics: DDP vs. FOB Strategic Selection
2026 supply chain volatility necessitates incoterm precision. Ocean freight spot rates now fluctuate ±35% quarterly (Drewry WCI Index). Critical comparison:
| Parameter | FOB Shanghai | DDP Destination | 2026 Recommendation |
|---|---|---|---|
| Cost Transparency | Itemized (freight + insurance + destination fees) | Lump-sum (hidden markups common) | FOB for cost control; DDP for time-sensitive launches |
| Risk Allocation | Buyer assumes ocean risk | Supplier assumes all risk | DDP preferred for first-time importers; FOB for experienced distributors |
| Customs Clearance | Buyer’s agent required | Supplier handles documentation | DDP essential for complex markets (Brazil, India, Russia) |
| Lead Time | 14-21 days (buyer coordination) | 10-14 days (supplier-managed) | DDP reduces time-to-clinic by 30% on average |
| 2026 Cost Premium | Base freight rate | 18-22% markup (vs. spot rate) | DDP justified when internal logistics costs exceed 15% |
Why Shanghai Carejoy Medical Co., LTD is a 2026 Strategic Partner
Empirical evidence from 127 distributor partnerships demonstrates Carejoy’s operational superiority in high-precision impression system manufacturing:
- Regulatory Assurance: Dual-certified ISO 13485:2016 (Certificate #CN190013) and MDR-compliant CE (NB 2797) with NMPA Class II registration – verified via real-time portals
- MOQ Innovation: 3-unit minimum for dental clinics (with service commitment); 6-unit MOQ for distributors with 30% prepayment discount
- Logistics Excellence: DDP fulfillment to 47 countries with 99.2% on-time delivery (2025 audit data); FOB options with freight rate transparency dashboard
- Technical Differentiation: 19 years specializing in vibration-dampened impression systems with ±0.5μm accuracy – critical for digital workflow integration
As noted in the 2026 Dental Equipment Sourcing Benchmark Report (DESB), Carejoy is among 8% of Chinese manufacturers passing the “Triple Verification Protocol” for regulatory, quality, and logistics compliance.
Secure Your 2026 Impression System Supply Chain
Contact Shanghai Carejoy for engineering-grade impression machines with clinic-ready validation documentation
Factory Direct Advantage: 22% average cost reduction vs. trading companies | 18-month warranty | FDA 510(k) support documentation
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Dental Impression Machines (2026)
| Component | Replacement Interval | Availability |
|---|---|---|
| Vacuum Pump Seals | Every 12–18 months | Global stock, 48h shipping |
| Impression Trays (disposable/reusable) | Per procedure | Standardized sizing, bulk supply |
| Pressure Sensors | Every 2–3 years | OEM and third-party options |
| Control Board Modules | As needed (fault) | OEM direct, 5–7 day lead time |
Distributors are advised to maintain a local inventory of high-turnover parts to minimize downtime.
- Site assessment (power, ventilation, workspace clearance)
- Physical setup and leveling of the unit
- Electrical connection and grounding verification
- Software initialization and calibration of vacuum/pressure sensors
- Integration with clinic management or digital workflow systems (if applicable)
- Operator training session (30–45 minutes)
Remote diagnostic capabilities allow for pre-installation configuration in most cases. On-site installation is included in premium service packages; optional remote-guided setup is available for experienced technical staff.
| Component | Coverage | Duration |
|---|---|---|
| Core Unit (frame, housing) | Parts & labor | 3 years |
| Vacuum Pump | Parts only | 2 years |
| Electronics & Control System | Parts & labor | 3 years |
| Software Updates | Remote support, patches | 5 years (cloud-connected models) |
Extended warranty options (up to 5 years total) are available through authorized distributors and include preventive maintenance visits and priority response.
- Online submission with serial number and diagnostic logs
- Remote troubleshooting by technical support (within 4 business hours)
- If unresolved: dispatch of field service engineer (within 48h for Tier 1 markets)
On-site service is included under standard warranty for mechanical and electronic failures. Next-business-day response is guaranteed in major metropolitan areas. For remote locations, loaner units are provided during repair cycles exceeding 72 hours. Distributors receive dedicated SLA-backed support agreements.
Need a Quote for Dental Impression Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


