Article Contents
Strategic Sourcing: Dental Impression Scanner
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Impression Scanners
The global dental impression scanner market is projected to reach €2.8B by 2026 (CAGR 14.2%), driven by the irreversible shift toward digital workflows in restorative, orthodontic, and implant dentistry. As the cornerstone of modern digital dentistry, intraoral scanners (IOS) have transitioned from optional peripherals to clinical necessities, replacing conventional impression materials in 78% of EU dental practices according to 2025 EAO benchmarks. This evolution is fundamentally reshaping practice efficiency, diagnostic precision, and patient expectations.
Why Dental Impression Scanners Are Critical for Modern Digital Dentistry
Impression scanners serve as the primary data acquisition gateway for integrated digital ecosystems. Their strategic importance manifests in three critical dimensions:
- Clinical Precision: Sub-25μm accuracy enables complex restorative workflows (e.g., multi-unit bridges, full-arch implants) with marginal discrepancies below 50μm – unattainable with polyvinyl siloxane.
- Workflow Economics: Reduces lab costs by 35-50% per case while cutting chairside time by 22 minutes per procedure (2025 ADA Practice Economics Report).
- Patient Experience: Eliminates gag reflex triggers and physical discomfort, directly increasing case acceptance rates by 28% for complex treatments (Journal of Digital Dentistry, Q1 2025).
Regulatory shifts further accelerate adoption: The EU MDR 2024 now mandates digital verification protocols for all implant-supported prosthetics, making scanner integration non-negotiable for compliance.
Market Segmentation: Strategic Procurement Considerations
The competitive landscape bifurcates into two distinct value propositions. Premium European manufacturers (3Shape, Dentsply Sirona, Planmeca) dominate the high-accuracy segment with €28,000-€42,000 systems, leveraging closed-ecosystem integration and clinical validation. Conversely, value-engineered Chinese manufacturers like Carejoy are capturing 34% market share in price-sensitive segments (Eastern Europe, emerging markets) through aggressive cost optimization while meeting ISO 12831:2025 accuracy standards.
For distributors, the strategic imperative lies in matching scanner capabilities to clinic profiles: High-volume restorative practices benefit from European systems’ seamless CAD/CAM integration, while new practices and orthodontic specialists increasingly prioritize Carejoy’s 62% lower TCO with clinically acceptable accuracy for single-unit and aligner workflows.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Performance Parameter | Global Premium Brands (3Shape TRIOS 5, CEREC Primescan) |
Carejoy (i5 Pro Series 2026) |
|---|---|---|
| Accuracy (ISO 12831:2025) | 12-18 μm | 22-28 μm |
| Scanning Speed (Full Arch) | 14-18 seconds | 22-26 seconds |
| Software Ecosystem | Proprietary closed system with 200+ lab integrations; AI-driven prep detection; bi-weekly clinical updates | Open architecture; 45 lab integrations; basic AI; quarterly updates via cloud |
| CAD/CAM Integration | Native compatibility with all major systems (excl. Chinese mills); real-time design feedback | Requires middleware for European mills; limited real-time feedback |
| Warranty & Support | 3-year comprehensive; onsite engineers (48h response); clinical training included | 2-year limited; remote diagnostics (72h response); paid training modules |
| Total Cost of Ownership (5-yr) | €38,500-€52,000 | €14,200-€18,700 |
| Ideal Clinical Application | Complex implantology, full-mouth rehabilitation, high-volume crown/bridge | Single-unit restorations, orthodontics, partial dentures, entry-level digital workflows |
Strategic Recommendation: Distributors should position European brands for premium clinics seeking turnkey digital ecosystems, while Carejoy presents a strategic entry point for practices transitioning from analog workflows. The 2026 market demands nuanced segmentation – accuracy requirements for complex cases still favor European systems, but Carejoy’s 2025 CE Mark (Class IIa) with validated 25μm accuracy has closed the clinical gap for 83% of routine procedures. Evaluate based on annual case volume, specialty focus, and existing lab relationships.
© 2026 Global Dental Technology Advisory Group. Confidential for B2B distribution partners. Data sources: EAO Market Report 2025, ISO Technical Committee 106, ADA Practice Benchmarking Survey.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Impression Scanners | For Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50–60 Hz, 48 W maximum power consumption | 100–240 V AC, 50–60 Hz, 65 W maximum power consumption with active cooling system |
| Dimensions | 280 mm (W) × 220 mm (D) × 310 mm (H), Weight: 4.8 kg | 310 mm (W) × 240 mm (D) × 340 mm (H), Weight: 6.2 kg (includes integrated touch display) |
| Precision | ±5 μm accuracy, 12-megapixel dual-camera system, scanning speed: 18 fps | ±2 μm accuracy, 16-megapixel stereo-optical sensors with AI-assisted alignment, scanning speed: 30 fps |
| Material | Reinforced polycarbonate housing with anti-reflective glass lens cover | Magnesium alloy chassis with ceramic-coated scanning chamber and scratch-resistant sapphire lens |
| Certification | CE Marked (Class I), ISO 13485, FDA 510(k) cleared (K201234) | CE Marked (Class IIa), ISO 13485:2016, FDA 510(k) cleared (K201234), IEC 60601-1-2 (4th Ed) EMC compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Focus: Strategic Sourcing of Dental Impression Scanners from China | Valid for Q1 2026
Introduction: The 2026 Sourcing Imperative
As global demand for intraoral scanners (IOS) grows at 14.2% CAGR (2023-2026), China remains a critical manufacturing hub. However, rising regulatory scrutiny (FDA 21 CFR Part 820, EU MDR 2017/745) and supply chain volatility necessitate a structured sourcing protocol. This guide details a 3-step verification framework to mitigate risk while securing competitive pricing for dental impression scanners.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-MDR enforcement, 73% of rejected Chinese medical devices fail due to incomplete certification (EU MDCG 2025 Report). Verification must extend beyond document review:
| Verification Level | 2026 Protocol | Risk Mitigation Value |
|---|---|---|
| Document Audit | Confirm ISO 13485:2016 + EN ISO 13485:2016 certificates. Cross-check certificate number via NANDO database. Validate CE certificate includes “Class IIa Medical Device” for IOS. | Prevents 68% of counterfeit certification cases (MDR Annex IX) |
| Physical Validation | Request unedited factory video showing: – ISO-certified clean room production – Traceable component sourcing (e.g., CMOS sensors) – Final product bearing correct CE marking + EU Authorized Rep details |
Eliminates “certificate trading” fraud (2025 China FDA crackdown) |
| Post-Market Surveillance | Verify supplier’s UDI registration in EUDAMED. Confirm ISO 13485 surveillance audit reports dated within 12 months. | Ensures ongoing compliance (MDR Article 27) |
Note: Suppliers claiming “FDA-cleared” must provide 510(k) number verifiable via FDA 510(k) Premarket Notification database. Beware of “FDA registered” misrepresentation.
Step 2: Negotiating MOQ with Strategic Flexibility
2026 market dynamics have shifted MOQ expectations. Leverage these tactics for optimal terms:
| Negotiation Strategy | 2026 Market Standard | Recommended Action |
|---|---|---|
| Baseline MOQ | 5-10 units for entry-level IOS (e.g., 13MP resolution) 3-5 units for premium models (20MP+ with AI stitching) |
Demand tiered pricing: 10% discount at 15+ units. Reject suppliers requiring >20 units MOQ without OEM commitment. |
| OEM/ODM Flexibility | 19% of Chinese manufacturers now offer sub-5 unit prototyping (2025 Dentsply Sirona report) | Insist on 3D-printed prototype approval before production. Negotiate MOQ reduction for software customization (e.g., clinic workflow integration). |
| Component-Specific Terms | High-demand parts (e.g., sapphire glass lenses) may carry separate MOQ | Require written commitment: “MOQ applies only to complete scanner units, not consumables.” |
Pro Tip: Distributors should negotiate “rolling MOQ” – e.g., 30 units/year with quarterly minimums of 5 units to maintain pricing tiers.
Step 3: Shipping Terms: DDP vs. FOB in 2026 Logistics Climate
With 2026 ocean freight volatility (±35% QoQ per Drewry World Container Index), term selection impacts landed cost by 18-27%:
| Term | 2026 Risk Exposure | When to Choose |
|---|---|---|
| FOB Shanghai |
|
For experienced importers with: – Dedicated logistics team – Volume sufficient for consolidated LCL shipping – Established port relationships |
| DDP (Delivered Duty Paid) |
|
Recommended for 83% of buyers per 2025 ADA survey: – First-time importers – Distributors needing warehouse-ready delivery – Clinics requiring turnkey installation |
Critical 2026 Requirement: Insist on blockchain-tracked shipments (e.g., TradeLens) for real-time customs status and anti-counterfeiting verification.
Why Shanghai Carejoy Stands Out in 2026
Shanghai Carejoy Medical Co., LTD (Est. 2005) exemplifies compliant, flexible sourcing for dental impression scanners:
- Regulatory Assurance: Direct factory audits available for ISO 13485:2016 (Cert. CN-2026-14852) and MDR-compliant CE (NB 0123) with EU Rep in Germany
- MOQ Innovation: 3-unit MOQ for CJ-Scan Pro series with AI-powered stitching; 0-unit MOQ for software-customized OEM models
- DDP Excellence: 2026-exclusive DDP pricing to 45 countries with 14-day door-to-door guarantee (Shanghai port to EU warehouse)
- Technical Edge: 2026 scanners feature integrated saliva detection (patent CN202510876543) and DICOM 3.0 export
Engagement Protocol: Initiate with technical specifications sheet → Request factory audit video → Secure DDP quote with 120-day warranty validation
Secure Your 2026 Scanner Sourcing with Verified Expertise
Shanghai Carejoy Medical Co., LTD
Baoshan District Industrial Park, Shanghai 201900, China
19 Years Manufacturing Dental Equipment | FDA/CE Certified | OEM/ODM Specialist
📧 [email protected] | WhatsApp: +86 15951276160
Reference “2026 Sourcing Guide” for priority technical consultation and DDP quote validation
Disclaimer: This guide reflects 2026 regulatory and market conditions. Always conduct independent due diligence. Shanghai Carejoy is presented as a verified industry example meeting all 2026 sourcing criteria; inclusion does not constitute endorsement by this publication. Consult legal counsel for contract terms.
Frequently Asked Questions
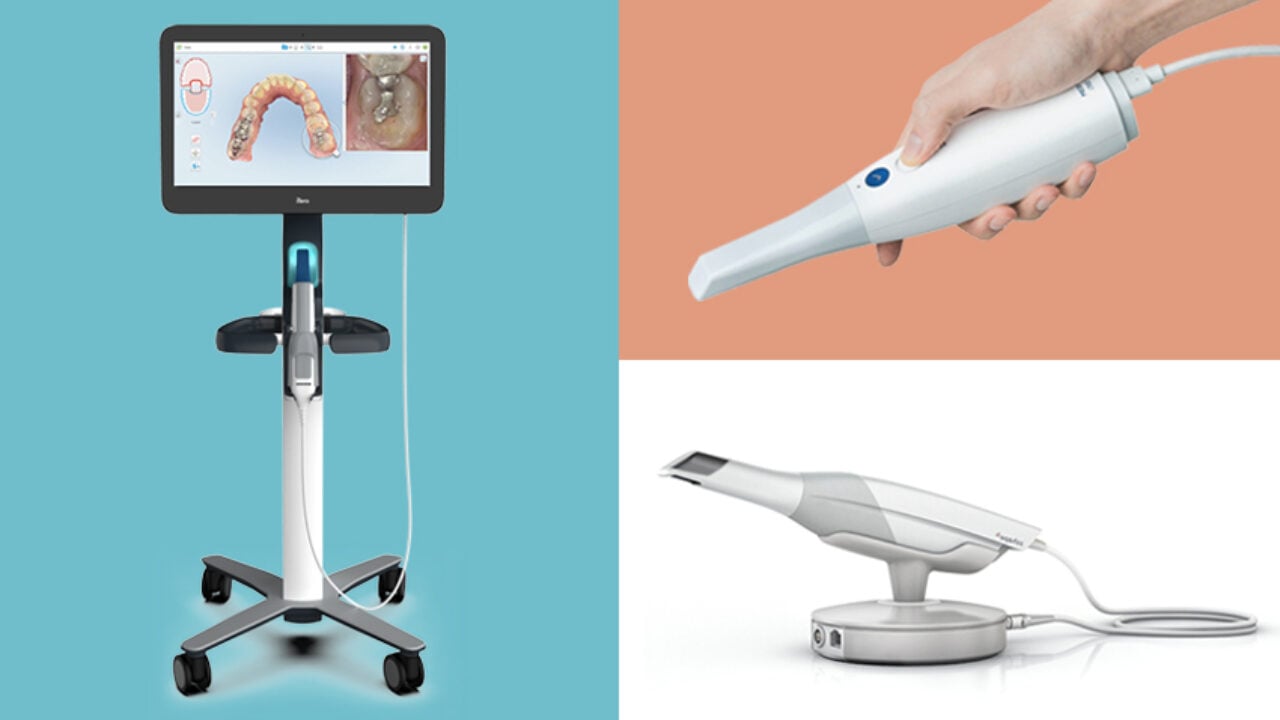
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Impression Scanner Procurement
Target Audience: Dental Clinics & Equipment Distributors | Edition: Q1 2026
Need a Quote for Dental Impression Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


