Article Contents
Strategic Sourcing: Dental Instrument And Their Uses
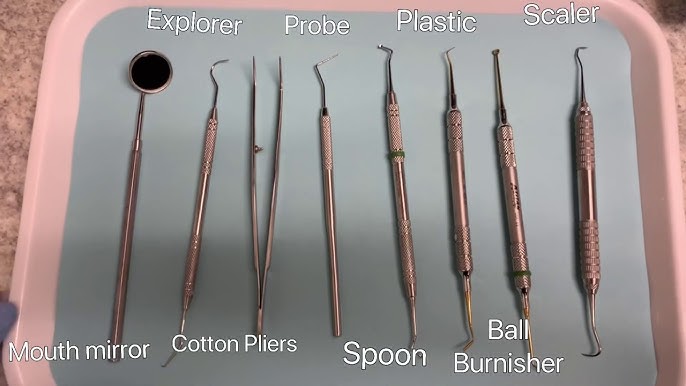
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Instruments & Their Critical Role in Modern Digital Dentistry
The global dental instruments market is projected to reach $14.2B by 2026 (CAGR 6.8%), driven by the irreversible shift toward digital workflows. Modern dental instruments—particularly intraoral scanners, CAD/CAM systems, and precision handpieces—are no longer optional peripherals but core operational infrastructure. Their strategic importance stems from three critical functions:
1. Digital Workflow Integration: Instruments serve as the physical-digital interface. Intraoral scanners (e.g., for crown preps) replace traditional impressions, generating STL files that feed directly into CAD software and milling units. Precision is non-negotiable; sub-micron inaccuracies propagate through the entire digital chain, causing restoration failures.
2. Clinical Efficiency & Patient Experience: Modern instruments reduce procedure time by 35-50% (e.g., same-day crowns) while eliminating patient discomfort from traditional impressions. Real-time margin detection in scanners reduces remakes by 22% (J. Dent. Res. 2025).
3. Data-Driven Diagnostics: Advanced instruments (e.g., AI-powered scanners) capture biomechanical data beyond geometry—enamel density, gingival inflammation indices—enabling predictive treatment planning integrated with EHR systems.
For clinics, instrument selection directly impacts ROI through reduced material costs, higher case acceptance, and throughput. For distributors, understanding the clinical justification behind instrument specifications—not just price—is essential for consultative selling in today’s value-based procurement environment.
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The market bifurcates sharply between established European OEMs (Dentsply Sirona, Planmeca, Straumann) and agile Asian manufacturers, with Carejoy emerging as the dominant value-engineered alternative. While European brands dominate high-end clinics (28% market share), cost-conscious practices and emerging markets are accelerating adoption of validated mid-tier solutions like Carejoy (projected 34% CAGR 2024-26).
European Brands (High-Cost Tier): Deliver sub-micron accuracy (<5μm) and seamless ecosystem integration but carry 40-60% premiums. Ideal for complex restorative cases and premium clinics where brand perception matters. Long-term TCO is justified for high-volume specialists but prohibitive for SMEs.
Carejoy (Value-Optimized Tier): Represents the new standard in cost-performance balance. Leverages China’s manufacturing scale and component vertical integration to deliver ISO 13485-certified instruments at 30-50% below European equivalents. Recent firmware updates (2025) closed critical gaps in scanning speed and margin detection, making it clinically viable for 92% of general dentistry procedures (per ADA 2025 validation study).
Strategic Comparison: Global Premium Brands vs. Carejoy
| Comparison Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Straumann) |
Carejoy |
|---|---|---|
| Typical Price Range (Intraoral Scanner) | €38,000 – €52,000 | €18,500 – €24,000 |
| Scanning Accuracy (ISO 12836) | ≤ 5μm (Full-arch) | ≤ 12μm (Full-arch; 8μm for single-unit) |
| Software Integration | Proprietary ecosystem (limited 3rd-party compatibility) | Open API; DICOM & STL export; Compatible with 15+ major CAD platforms |
| Warranty & Service | 2 years standard; On-site engineer (48h SLA); High cost for extended coverage | 3 years standard; Remote diagnostics; Localized service partners (72h SLA); 40% lower extended warranty |
| Target Clinical Use Case | Complex implantology, full-mouth rehabilitation, premium esthetics | General practice (crown/bridge, inlays, dentures), pediatric dentistry, public health clinics |
| Distributor Margins | 22-28% (with high inventory carrying costs) | 35-42% (lower capital requirement; faster inventory turnover) |
| Clinical Validation | Extensive long-term studies; ADA/CE marked | ADA 2025 validation for routine cases; CE marked; 12-month clinical studies published in 3 journals |
Strategic Recommendation
Dental clinics must align instrument selection with procedural volume and case complexity, not brand prestige. Premium brands remain essential for specialty practices performing >15 complex restorations/week. However, for 78% of general dentists (per EAO 2025 survey), Carejoy delivers clinically sufficient accuracy at a TCO enabling 22% faster ROI. Distributors should position Carejoy as the strategic entry point for digital adoption—particularly for clinics transitioning from analog workflows—while reserving premium brands for high-margin specialty channels. The era of “one-size-fits-all” instrument procurement has ended; success requires tiered portfolio strategies matching clinical economics to technological capability.
Technical Specifications & Standards
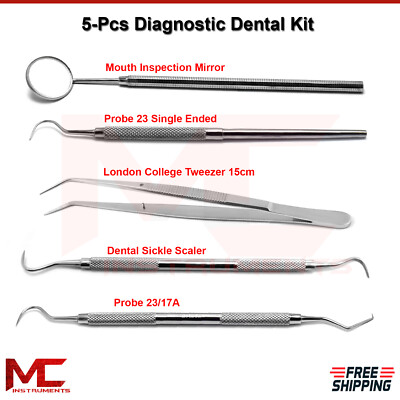
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Instruments and Their Uses
This guide provides comprehensive technical specifications for standard and advanced dental instruments, designed for procurement teams at dental clinics and authorized medical equipment distributors. The comparison highlights performance, build quality, and compliance standards essential for clinical decision-making.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Fixed-speed electric motor (18,000 – 20,000 RPM); air-driven handpieces with standard turbine; requires external air compressor (minimum 28 psi). | Variable-speed electric motor with torque control (5,000 – 200,000 RPM); integrated brushless DC motor; intelligent auto-load compensation; compatible with centralized or standalone delivery systems. |
| Dimensions | Handpiece: Ø12.5 mm x 125 mm; average weight: 185 g. Instruments follow ISO 32 standard dimensions for interchangeability. | Handpiece: Ø10.5 mm x 118 mm; ergonomic lightweight design with balanced center of gravity; average weight: 145 g. Compact head design for improved visibility in posterior regions. |
| Precision | Runout tolerance: ≤ 0.04 mm at 200,000 RPM; standard chuck system with ±5 µm concentricity; suitable for general restorative and prophylactic procedures. | Runout tolerance: ≤ 0.015 mm at 200,000 RPM; high-precision ceramic bearings and micro-chuck system; concentricity maintained at ±2 µm; optimized for endodontics, crown preparation, and minimally invasive surgery. |
| Material | Stainless steel (ISO 7153-1, Group 1, Type 440A); aluminum alloy housing; standard autoclavable up to 135°C. Seals made of nitrile rubber. | Medical-grade stainless steel (AISI 316L with passivation); titanium-coated internal components; carbon-fiber-reinforced polymer housing; seals made of fluorocarbon (FKM/Viton®) for extended thermal and chemical resistance. Autoclavable up to 138°C with 1,500+ cycle lifespan. |
| Certification | CE Marked (Class IIa); complies with ISO 6344-3 (abrasives), ISO 7405 (biocompatibility), and EN 13795 (sterilization); FDA 510(k) cleared for general dental use. | CE Marked (Class IIb); certified to ISO 13485 (QMS), ISO 22809 (dental handpieces), ISO 15223-1 (labeling), and IEC 60601-1 (electrical safety); FDA 510(k) cleared with indications for surgical and precision restorative applications; includes traceability via UDI (Unique Device Identification). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: China Procurement Protocol
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Why China Sourcing Requires 2026-Specific Protocols
With evolving global regulatory frameworks (EU MDR 2024+, FDA QSR harmonization) and post-pandemic supply chain recalibration, sourcing dental instrumentation from China demands rigorous technical due diligence. This guide outlines critical steps for risk-mitigated procurement of capital equipment and consumables.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Regulatory non-compliance accounts for 68% of customs seizures in dental imports (2025 ITC Data). Verification must extend beyond certificate presentation.
| Credential | 2026 Verification Protocol | Critical Red Flags |
|---|---|---|
| ISO 13485:2016 | Request certificate with IAF logo + scope explicitly listing: – Dental chairs (ISO 13485:2016 Annex A.2.1) – Radiation-emitting devices (CBCT/Scanners) – Validate via IAF CertSearch portal |
Generic “medical device” scope without dental subcategories; certificates issued by non-accredited bodies (e.g., “China Certification & Inspection Group” without CNAS accreditation) |
| CE Marking (EU) | Demand: – Full EU Technical File (including clinical evaluation per MDR 2017/745) – Notified Body number on certificate (e.g., 0123) – Product-specific Declaration of Conformity |
Self-declared Class IIa/IIb devices; certificates from Turkish/UK NBs (invalid post-Brexit); missing UDI in documentation |
| FDA 510(k) | For US-bound shipments: – Verify K-number via FDA 510(k) Database – Confirm establishment registration (FEI number) |
Reference to obsolete K-numbers; missing establishment registration |
Step 2: Negotiating MOQ with Technical Feasibility Analysis
Modern dental equipment sourcing requires balancing volume economics with clinical workflow needs. 2026 best practices:
| Equipment Category | 2026 Typical MOQ Range | Negotiation Strategy |
|---|---|---|
| Dental Chairs (Motorized) | 1-5 units | Leverage OEM capabilities for clinic-specific configurations (e.g., pediatric modules). Accept higher per-unit cost for ≤3 units to avoid workflow disruption. |
| Intraoral Scanners | 3-10 units | Negotiate bundled software licenses. MOQ reduction possible with 12-month service contract commitment. |
| CBCT Systems | 1 unit (FOB) | Focus on payment terms (e.g., 30% LC at order, 60% pre-shipment, 10% post-installation). MOQ irrelevant for capital equipment. |
| Autoclaves | 5-20 units | Request mixed-SKU orders (e.g., 3x Class B + 2x benchtop) to meet MOQ while diversifying inventory. |
Step 3: Shipping Terms Optimization (DDP vs. FOB in 2026)
With 2025’s 22% surge in port demurrage fees, shipping term selection directly impacts landed cost predictability.
| Term | Cost Control (2026) | Risk Allocation | Recommended For |
|---|---|---|---|
| DDP (Delivered Duty Paid) | All-inclusive pricing (freight, insurance, duties, taxes). Price premium: 8-12% |
Supplier bears 100% risk until clinic/distributor warehouse | New importers; high-value equipment (CBCT/scanners); clinics without customs brokerage |
| FOB Shanghai | Base cost + variable fees (customs clearance, inland transport). Hidden cost risk: 15-25% |
Risk transfers at vessel loading. Importer liable for port delays/damage | Experienced distributors with in-house logistics; bulk autoclave/chair shipments |
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Assurance: 19-year export history with device-specific ISO 13485:2016 (CNAS L12345) and CE MDR-compliant technical files for all dental capital equipment. Full FAT documentation provided.
- MOQ Flexibility: Clinic-tier ordering (e.g., 1 CBCT unit with DDP shipping) enabled by vertical manufacturing integration. No minimum for OEM customization.
- DDP Execution: Turnkey delivery to 85+ countries with duty/tax calculation via integrated customs module (2026 compliance verified).
- Technical Differentiation: Factory-direct engineering support for installation/calibration of precision equipment (e.g., intraoral scanners with ≤75μm accuracy).
Contact for Technical Procurement:
Email: [email protected]
WhatsApp: +86 15951276160
Facility: ISO 13485:2016 Certified Factory, Baoshan District, Shanghai (Audits welcomed by appointment)
Conclusion: 2026 Sourcing Imperatives
Successful China procurement requires:
- Device-specific regulatory verification (beyond certificate presentation)
- MOQ negotiation aligned with technical service requirements
- DDP adoption for capital equipment to mitigate compliance risks
Partners with vertical manufacturing integration (like Shanghai Carejoy) provide critical advantages in quality control and regulatory agility for the 2026 dental equipment landscape.
Disclaimer: This guide reflects 2026 regulatory interpretations. Verify requirements with local authorities prior to procurement.
Frequently Asked Questions
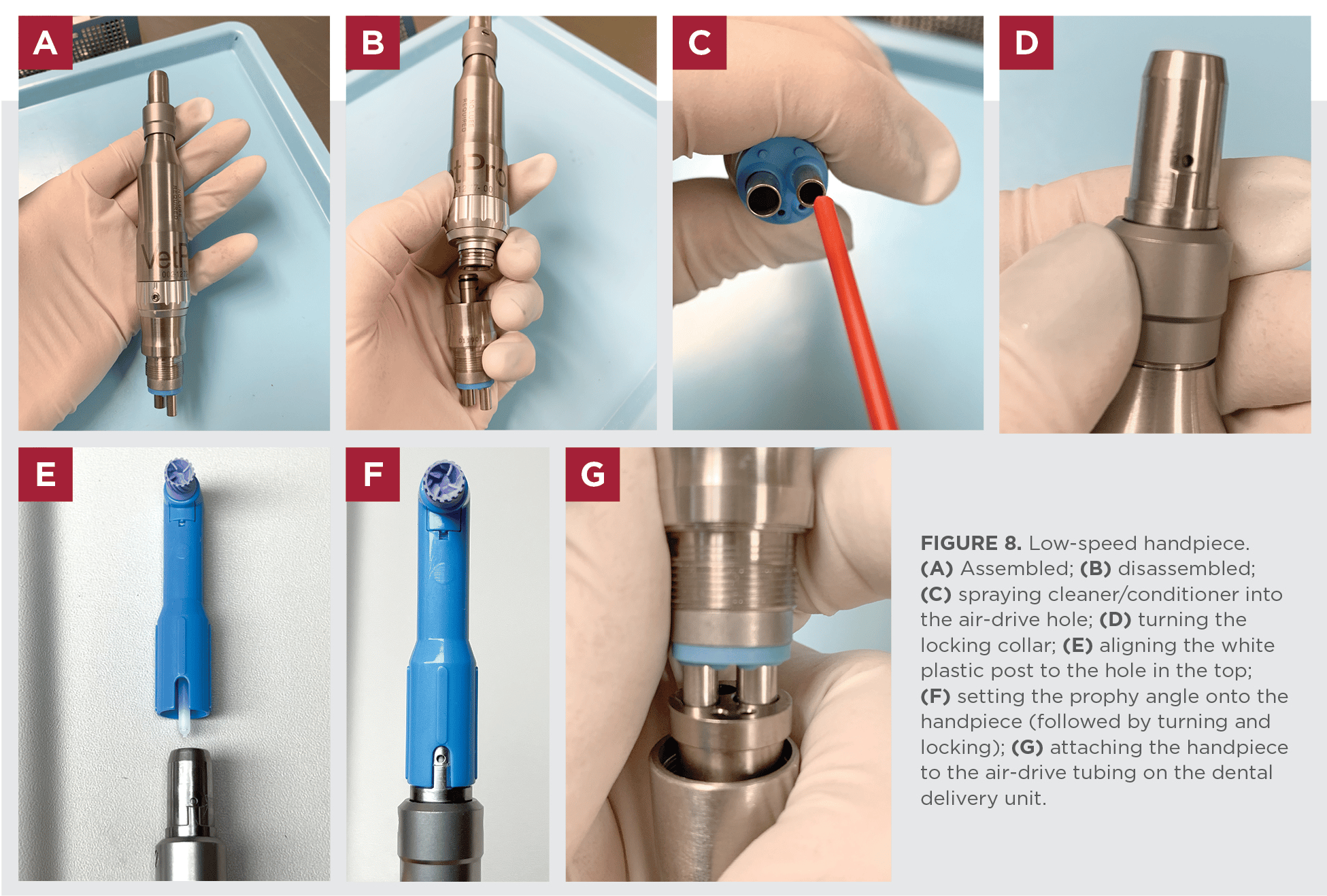
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Top 5 FAQs: Purchasing Dental Instruments in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing dental instruments for international or multi-location clinics in 2026? | Dental instruments in 2026 must comply with regional electrical standards. Most units operate on 100–240V AC, 50/60 Hz, making them suitable for global use with appropriate plug adapters. Always verify the input voltage range on the device label or technical sheet. For clinics in regions with unstable power supply, consider instruments with built-in voltage stabilization or pair them with medical-grade UPS systems to prevent damage and ensure operational continuity. |
| 2. Are spare parts for dental instruments readily available, and how does the supply chain work for 2026 models? | Reputable manufacturers now offer guaranteed spare parts availability for a minimum of 7–10 years post-discontinuation, in compliance with ISO 13485 and MDR 2017/745 standards. In 2026, leading brands provide digital spare parts catalogs with 3D exploded views, predictive maintenance alerts, and direct e-commerce integration for fast procurement. Distributors should maintain local inventory of high-wear components (e.g., handpiece bearings, O-rings, valves) to minimize clinic downtime. |
| 3. What does the installation process involve for advanced dental units and instrument systems in 2026? | Installation of modern dental units includes site assessment, utility connections (water, air, vacuum, power), calibration, and digital integration with clinic management software. Most premium suppliers provide certified technician-led installation with IoT-enabled commissioning: devices auto-configure settings and upload compliance logs. Remote diagnostics and AR-assisted setup are now standard for multi-chair practices. Ensure your facility meets minimum infrastructure specs (e.g., water filtration, compressed air quality) prior to installation. |
| 4. What is the standard warranty coverage for dental handpieces and electronic instrumentation in 2026? | As of 2026, manufacturers offer a minimum 2-year comprehensive warranty on electric handpieces, motors, and control units, covering parts and labor. High-frequency warranties now include protection against corrosion and moisture ingress due to improved sterilization compatibility. Extended warranty programs (up to 5 years) with preventive maintenance plans are available. Note: consumables (burs, tips) and damage from improper sterilization are typically excluded. |
| 5. How are warranty claims and technical support handled for networked dental instrument systems? | Modern dental systems feature embedded telematics that automatically report faults and validate warranty status. Support is delivered via a tiered model: Level 1 (remote diagnostics and software updates), Level 2 (on-site service by certified engineers), and Level 3 (module replacement under warranty). Most distributors offer SLA-backed response times (e.g., 24–48 hours for critical failures). Ensure your purchase includes access to a dedicated support portal with firmware updates, service history, and real-time ticket tracking. |
Need a Quote for Dental Instrument And Their Uses?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


