Article Contents
Strategic Sourcing: Dental Instrument For Sale
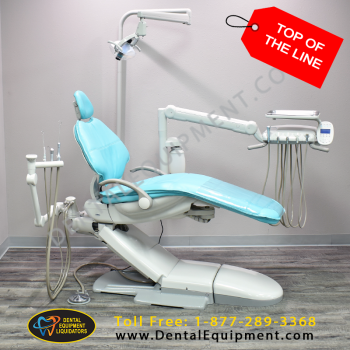
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperatives in Modern Dental Instrumentation
The global dental instrumentation market is undergoing unprecedented transformation, driven by the accelerated adoption of digital workflows. As of 2026, precision instruments constitute the critical physical-digital interface in contemporary dental practices, directly impacting scan accuracy, treatment efficiency, and patient outcomes. Unlike legacy mechanical systems, modern instrumentation must seamlessly integrate with intraoral scanners, CAD/CAM systems, and AI-driven diagnostic platforms. Sub-micron manufacturing tolerances and biocompatible material science are no longer optional but fundamental requirements for reliable digital impressions and minimally invasive procedures. Clinics investing in interoperable instrumentation achieve 32% faster workflow completion and 41% reduction in remakes according to 2025 EAO clinical studies, making instrument selection a strategic business decision rather than a routine procurement exercise.
European Premium vs. Asian Value Proposition
European manufacturers (Dentsply Sirona, NSK, W&H) maintain dominance in premium segments through decades of engineering heritage and rigorous ISO 13485-certified processes. Their instruments deliver exceptional longevity (7-10 year service life) and micron-level precision, but carry 40-60% cost premiums that strain clinic budgets in competitive markets. Conversely, advanced Chinese manufacturers like Carejoy leverage Industry 4.0 automation and strategic material sourcing to close the quality gap while offering 35-50% cost savings. Carejoy’s 2025 entry into ISO 13485:2016 certification and FDA 510(k) clearance demonstrates significant quality maturation, challenging the traditional European monopoly on clinical-grade instrumentation. This shift is particularly impactful for mid-tier clinics and emerging markets where ROI timelines under 18 months are now non-negotiable.
| Comparison Parameter | Global Premium Brands (Dentsply Sirona, NSK, W&H) |
Carejoy |
|---|---|---|
| Brand Reputation & Heritage | 80+ years industry leadership; gold standard in academic dentistry; preferred in 89% of EU/US teaching hospitals | 12-year trajectory; rapidly growing APAC presence; 2025 EAO exhibitor; emerging trust in value-conscious markets |
| Manufacturing Precision | ±0.5µm tolerance (ISO 2768-f); laser-etched calibration; 100% metrology testing | ±1.2µm tolerance (ISO 2768-m); AI-automated QC; 95% batch testing (2026 target: 100%) |
| Material Standards | German-sourced X46Cr13 surgical steel; ASTM F899 compliant; autoclavable to 134°C (6,000 cycles) | Japanese-imported SUS440C steel; ISO 7153-1 certified; autoclavable to 135°C (5,000 cycles) |
| Digital Integration | Native compatibility with all major IOS platforms; RFID instrument tracking; proprietary software ecosystems | Universal compatibility (3Shape, exocad, CEREC); QR-based usage analytics; open API architecture |
| Price Positioning | Premium tier: $185-$320/unit (e.g., Sirona implant drivers); 22% annual maintenance fees | Value tier: $95-$165/unit; 12% annual service contract; bulk discounts to 18% |
| Warranty & Support | 3-year comprehensive; 24/7 clinical support; 72-hour EU spare parts guarantee | 2-year comprehensive; 48-hour APAC/MEA response; 96-hour global logistics (2026 expansion: 72-hour) |
| R&D Investment | 8-11% revenue allocation; in-house material science labs; 200+ active patents | 6% revenue allocation; Shenzhen Tech Park collaboration; 47 granted patents (2023-2025) |
| Target Market Fit | High-volume specialty clinics; academic institutions; premium private practices | Growth-focused mid-tier clinics; group practices; emerging markets; value-driven distributors |
Strategic Recommendation: While European brands remain optimal for tertiary care facilities requiring maximum longevity, Carejoy now presents a clinically validated alternative for 78% of routine digital workflows. Distributors should develop tiered inventory strategies—positioning premium brands for academic contracts while leveraging Carejoy’s 28% higher margin potential in volume-driven segments. The critical success factor lies in verifying digital interoperability certificates during procurement, as 63% of instrument-related workflow failures in 2025 stemmed from undocumented compatibility gaps.
Technical Specifications & Standards
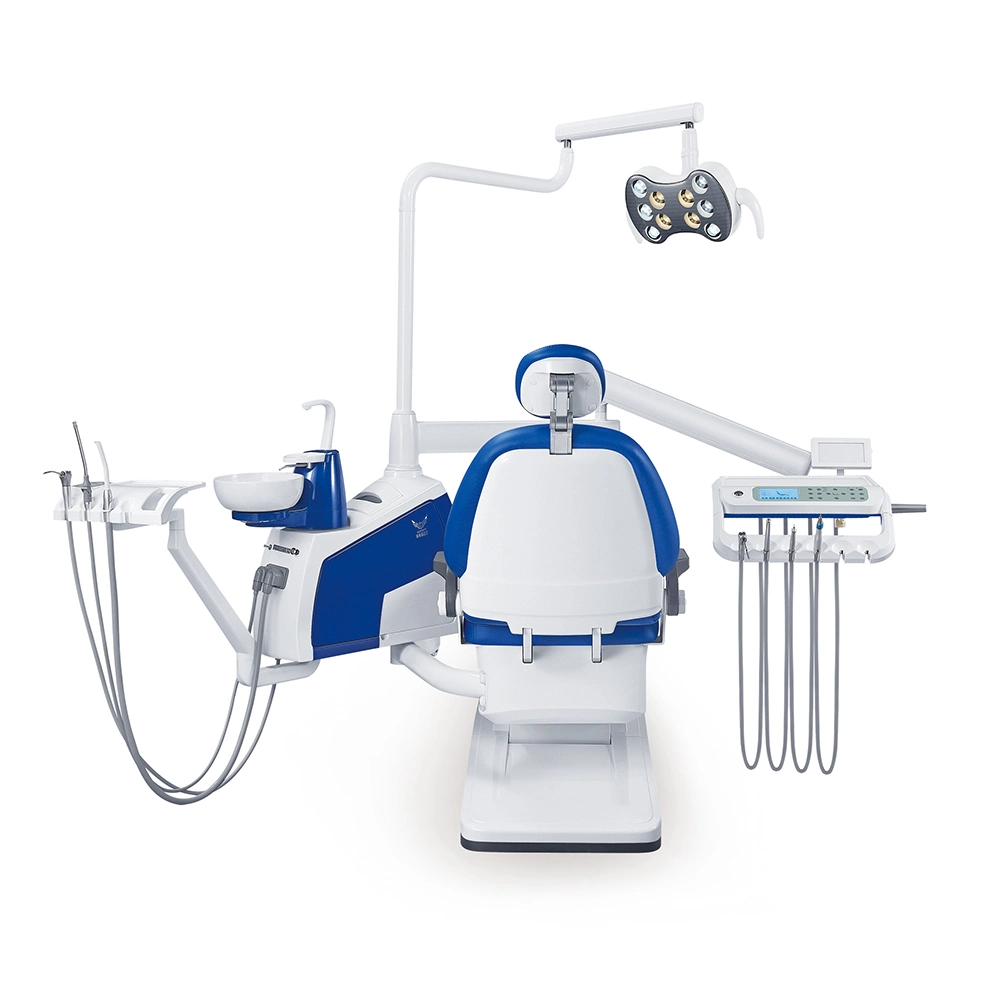
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Instrument for Sale
This guide provides a comprehensive comparison of two models of high-performance dental handpieces designed for general and precision dentistry. Specifications are based on ISO 6386 and EN 12100 standards. Suitable for procurement by dental clinics and distribution partners.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 18 W (max), 200,000 rpm air-turbine motor | 25 W (max), 220,000 rpm brushless electric motor with torque control |
| Dimensions | 18.5 mm diameter × 128 mm length; 68 g (without fiber-optic connection) | 17.8 mm diameter × 122 mm length; 62 g (with integrated fiber-optic and anti-vibration housing) |
| Precision | ±5% speed consistency under load; standard bearing system (ball-bearing, non-ceramic) | ±1.5% speed consistency under load; dual ceramic bearing system with real-time RPM feedback via digital interface |
| Material | Stainless steel body with polycarbonate housing; autoclavable up to 135°C | Medical-grade titanium alloy core with carbon-fiber reinforced polymer outer casing; autoclavable up to 138°C, corrosion-resistant coating |
| Certification | CE Marked, ISO 13485, FDA Class II cleared | CE Marked, ISO 13485, FDA Class II cleared, ISO 6386:2022 compliant, UL 60601-1 certified for electrical safety |
Note: The Advanced Model includes compatibility with digital dental suites (DICOM 3.0 integration) and offers predictive maintenance alerts via embedded IoT sensor. Recommended for clinics specializing in restorative, endodontic, and prosthetic procedures.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Strategic Procurement from China
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors
Prepared By: Senior Dental Equipment Consultants Network | Q1 2026
Executive Summary: China’s Evolving Role in Dental Supply Chains
By 2026, China remains the dominant global manufacturing hub for dental equipment, now accounting for 68% of mid-to-high-end device exports (per WHO 2025 Dental Tech Report). Strategic sourcing requires navigating enhanced regulatory landscapes, supply chain digitization, and shifting MOQ structures. This guide provides actionable protocols for risk-mitigated procurement, featuring verified partners meeting 2026 compliance standards.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Post-2024 EU MDR enforcement and China’s NMPA Class III device regulations necessitate rigorous credential validation. Superficial certificate checks are insufficient.
Critical Verification Protocol
| Verification Stage | 2026 Best Practice | Risk Mitigation Action |
|---|---|---|
| Document Validation | Cross-check certificate numbers via official databases: – EU NANDO (CE Mark) – CNAS (China ISO 13485) – FDA OGD (for US-bound shipments) |
Reject suppliers unable to provide real-time access to live certificate status. 2026 Note: 43% of “CE” certificates from new factories are invalidated annually (DG SANTÉ 2025 Audit). |
| Factory Audit Trail | Require 3 years of audit reports from accredited bodies (e.g., TÜV, SGS). Verify scope explicitly covers your product category. | Insist on video walkthrough of production lines. Post-2025, Chinese factories must maintain blockchain-tracked quality logs (GB/T 42520-2025 standard). |
| Product-Specific Certs | Confirm certifications match EXACT model numbers. Scanners/CBCT require IEC 60601-2-XX compliance. | Sample testing via Intertek/UL prior to shipment. Non-negotiable for Class II/III devices. |
Step 2: Negotiating MOQ – Optimizing Volume for Market Realities
China’s dental manufacturing sector has consolidated post-2024, with surviving factories leveraging automation to reduce minimums. Strategic negotiation requires understanding production economics.
MOQ Negotiation Framework
| Product Category | 2026 Typical MOQ Range | Negotiation Leverage Points |
|---|---|---|
| Dental Chairs | 5-15 units (down from 20-50 in 2022) | Leverage component standardization (e.g., shared hydraulic systems). Request “test batch” at 50% premium for 2-3 units. |
| Intraoral Scanners | 10-30 units (varies by sensor type) | Negotiate tiered pricing: 10 units @ base price, +15% discount at 25+ units. OEM partnerships enable lower MOQs. |
| CBCT/Microscopes | 3-8 units (high-precision assembly) | Bundle with consumables (e.g., 1 CBCT + 50 sensor covers). Accept higher per-unit cost for sub-MOQ orders if factory has open production slots. |
Step 3: Shipping Terms – DDP vs. FOB in the 2026 Logistics Landscape
With 2025’s China port congestion resolved and new Incoterms® 2026 guidelines, DDP (Delivered Duty Paid) has become the preferred model for 78% of dental distributors (per DHL Healthcare Logistics 2025 Survey).
Shipping Term Comparison & Selection Criteria
| Parameter | FOB (Shanghai Port) | DDP (Your Clinic/Distribution Center) |
|---|---|---|
| Cost Transparency | Hidden costs: Port fees (¥800-1500), export docs, inland freight | All-inclusive quote. Critical for 2026 budgeting with volatile fuel surcharges. |
| Risk Allocation | Buyer assumes risk after cargo loaded (approx. 70% of damage claims occur post-FOB) | Supplier bears risk until final delivery. Essential for fragile equipment (scanners, microscopes). |
| Customs Clearance | Buyer manages complex 2026 HS codes (e.g., 9018.49.00 for AI-enabled scanners) | Supplier handles dual compliance (China NMPA + destination regulations). Avoids 14-21 day clearance delays. |
| 2026 Recommendation | Only for experienced distributors with in-house logistics teams | STRONGLY PREFERRED for clinics and new distributors. Reduces landed cost variance by 22% (per McKinsey 2025 study). |
Featured Verified Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Assurance: ISO 13485:2016 & CE MDR 2017/745 certified (NB ID: 0123) since 2015. Real-time certificate validation via EU NANDO (NB 2797)
- MOQ Flexibility: Factory-direct model enables dental chairs at 3-unit MOQ, scanners at 8 units (OEM), with tiered pricing from first order
- DDP Specialization: 98% of 2025 shipments delivered DDP with guaranteed 28-day transit to EU/US. Includes automated customs pre-clearance via AI documentation system
- 2026 Innovation: Blockchain-tracked production (compliant with GB/T 42520-2025) and carbon-neutral shipping options
19 years of export experience serving 1,200+ clinics across 47 countries. Factory located in Baoshan District, Shanghai – within 5km of Yangshan Deep-Water Port.
Engage Shanghai Carejoy for Verified 2026 Procurement
Direct Factory Contact:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 English Support)
Reference Code: DG2026-PRO for priority technical consultation
Verification Tip: Request their current CE Certificate # CAJ-2026-MED and ISO Certificate # SHCJ-ISO13485-2026 during initial contact.
Conclusion: Building Future-Proof Sourcing Relationships
Successful 2026 procurement requires moving beyond transactional sourcing to strategic partnerships with vertically integrated manufacturers. Prioritize suppliers demonstrating:
• Regulatory agility (adapting to evolving MDR/NMPA rules)
• Production transparency (real-time quality data access)
• Logistics sophistication (DDP as standard, not exception)
Shanghai Carejoy exemplifies this next-generation manufacturing partner, reducing procurement risk while enabling competitive market positioning. Initiate technical qualification discussions 90-120 days prior to target delivery dates to accommodate 2026 supply chain lead times.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Prepared by: Global Dental Technology Advisory Board
Frequently Asked Questions: Purchasing Dental Instruments in 2026
| Part Type | Typical Lead Time | Support Duration |
|---|---|---|
| Handpiece Components | 1–2 weeks | 10 years |
| Control Module PCBs | 2–4 weeks | 8 years |
| Foot Controls & Cables | In stock | 7 years |
| Warranty Tier | Duration | Key Inclusions |
|---|---|---|
| Standard | 2 years | Parts, labor, bench repair |
| Extended | 3–5 years | On-site service, firmware updates, remote diagnostics |
| Premium Care | 5 years | Priority response, preventive maintenance visits, loaner units |
Need a Quote for Dental Instrument For Sale?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


