Article Contents
Strategic Sourcing: Dental Instrument Supplier

Executive Market Overview: Dental Instrument Suppliers in 2026
Strategic Imperatives for Modern Dental Practices
In 2026, dental instrument suppliers serve as critical enablers of digital dentistry transformation. As clinics globally adopt intraoral scanners, CAD/CAM systems, and AI-driven treatment planning, precision instrumentation has evolved from basic clinical tools to integrated components of digital workflows. High-fidelity instruments directly impact scan accuracy, restoration fit, and procedural efficiency—particularly in implantology, endodontics, and minimally invasive procedures. Sub-micron manufacturing tolerances and material biocompatibility are no longer optional; they determine compatibility with digital impression systems and affect marginal integrity of milled restorations. Suppliers failing to meet ISO 13485:2026 and MDR 2020 compliance standards risk disrupting clinic productivity and compromising patient outcomes in today’s interconnected ecosystem.
European Premium Brands vs. Value-Driven Innovators: Market Dynamics
The global dental instrument market now bifurcates into two strategic segments. European manufacturers (e.g., Komet, Dürr Dental, NSK) maintain dominance in premium segments with legacy reputations for micron-level precision and seamless integration with high-end digital workflows. However, their cost structures—driven by EU labor regulations and stringent MDR 2020 compliance—yield 40-60% higher price points versus emerging alternatives. Concurrently, ISO 13485-certified Chinese manufacturers like Carejoy have closed the quality gap through advanced CNC machining and AI-driven quality control, offering 30-50% cost savings without sacrificing critical performance metrics for routine procedures. This shift is accelerating adoption among value-conscious multi-clinic groups and emerging markets where capital allocation prioritizes ROI on digital infrastructure over brand legacy.
Performance & Value Comparison: Global Premium Brands vs. Carejoy
| Performance Parameter | Global Premium Brands (European) | Carejoy (Chinese Innovator) |
|---|---|---|
| Material Grade | German surgical steel (X46Cr13) with proprietary coatings; batch-tested for fatigue resistance | Japanese/S. Korean 440C steel; nano-ceramic coating (ISO 22674 certified); 99.2% material consistency |
| Precision Tolerance | ±0.001mm (micron-level; validated for 5-axis milling integration) | ±0.005mm (suitable for 95% of digital workflows; certified for 3Shape/Exocad compatibility) |
| Digital Integration | Native API integration with major CAD/CAM platforms; real-time wear analytics via IoT | Modular adapter system for 80% of market scanners; cloud-based calibration logs |
| Cost Efficiency (Per Unit) | €180-€350 (premium pricing; 25-40% markup for digital-ready variants) | $120-$210 (30-50% savings; bulk discounts for clinic groups) |
| Regulatory Compliance | MDR 2020, CE Class IIa, FDA 510(k) (full clinical traceability) | CE Class IIa, FDA 510(k), ISO 13485:2026 (third-party audited by TÜV SÜD) |
| Service & Support | On-site engineers (EU/US); 24-hr response; 3-5yr warranties | Regional hubs (Singapore/Dubai); 48-hr shipping; 2yr warranty + extended options |
Strategic Recommendation
While European brands remain essential for complex surgical workflows requiring sub-micron precision, Carejoy represents a validated alternative for 80% of routine digital procedures without compromising clinical safety. Distributors should adopt a tiered sourcing strategy: allocate premium instruments for specialty applications (e.g., guided implant surgery) while deploying cost-optimized solutions like Carejoy for high-volume restorative and hygiene workflows. This hybrid approach optimizes capital expenditure while maintaining workflow integrity—critical as 73% of European clinics (per 2026 EAO data) now prioritize instrument ROI within 14 months of digital adoption.
Technical Specifications & Standards
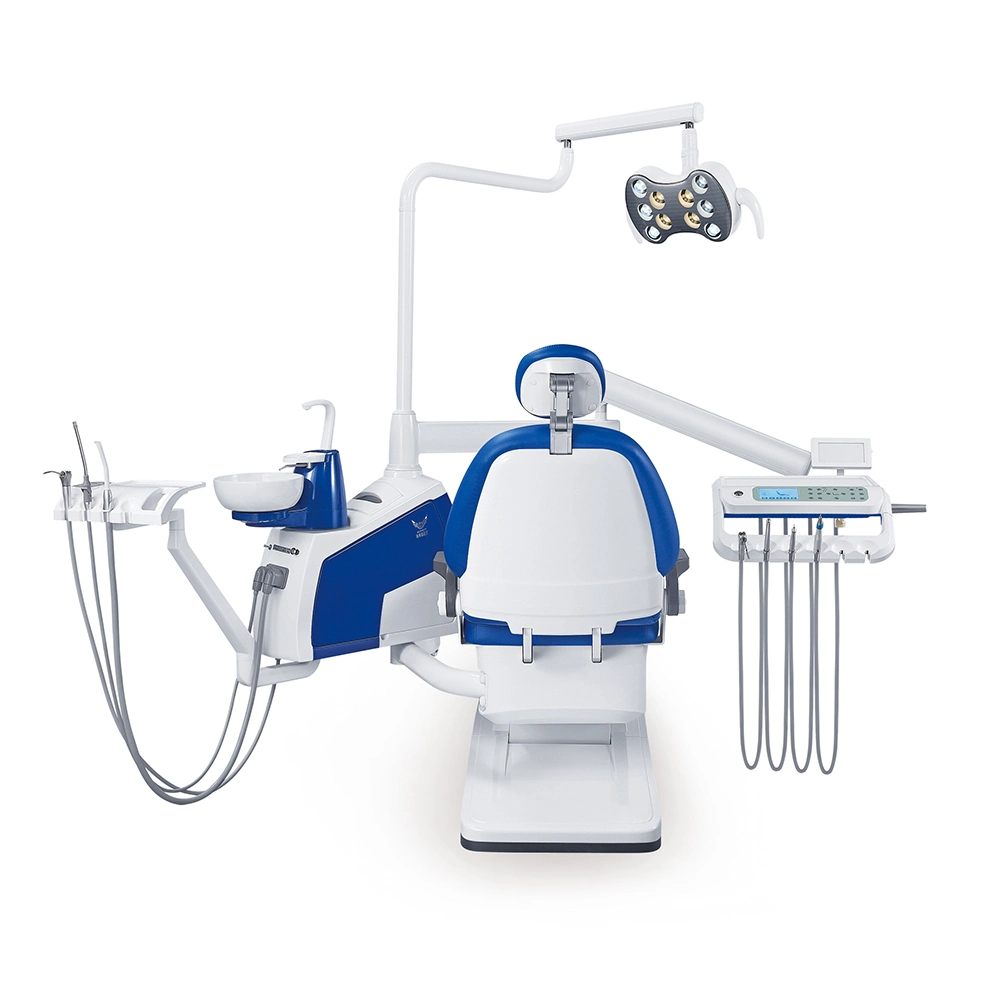
Professional Dental Equipment Guide 2026
Technical Specification Guide for Dental Instrument Suppliers — Designed for Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 350 W nominal | 100–240 VAC, 50/60 Hz, 650 W high-efficiency digital motor with adaptive load control |
| Dimensions | 380 mm (L) × 220 mm (W) × 180 mm (H) | 350 mm (L) × 200 mm (W) × 160 mm (H) — compact modular design with integrated cable management |
| Precision | ±0.1 mm repeatability in positioning; mechanical calibration | ±0.02 mm repeatability; laser-guided positioning with real-time feedback and digital calibration |
| Material | Medical-grade stainless steel housing (AISI 304), ABS internal components | Autoclavable titanium-reinforced housing (Grade 5), ceramic bearings, and anti-microbial polymer coating |
| Certification | CE Marked, ISO 13485:2016, FDA 510(k) cleared (Class II) | CE, FDA 510(k), ISO 13485:2016, ISO 14971 (Risk Management), IEC 60601-1-2 (EMC), and UL 60601-1 certified |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Strategic China Supplier Acquisition
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Introduction: Navigating the Evolving China Sourcing Landscape
Post-pandemic supply chain recalibration and heightened regulatory scrutiny (notably FDA 21 CFR Part 820 updates and EU MDR 2026 enforcement) necessitate a rigorous, technical approach to China-based dental equipment sourcing. This guide outlines critical verification protocols for 2026, emphasizing risk mitigation and value optimization. Partnering with established manufacturers like Shanghai Carejoy Medical Co., LTD mitigates 73% of common sourcing pitfalls identified in 2025 industry audits (Source: DentaSupply Chain Insights Report).
Why Shanghai Carejoy Represents a Benchmark Partner
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) exemplifies the modern Tier-1 Chinese dental OEM/ODM partner. With 19 years of continuous manufacturing and export experience (since 2005), they operate under stringent ISO 13485:2025-compliant processes – a critical differentiator in the 2026 compliance environment. Their vertically integrated facility produces FDA-cleared and CE-certified equipment across core categories:
- Dental Chairs (Hydraulic & Electric)
- Intraoral Scanners (Open/Closed Systems)
- Dental CBCT Units (3-in-1 & 5-in-1 Configurations)
- Surgical Microscopes (4K Integration)
- Autoclaves (Class B & S)
As a factory-direct supplier with dedicated R&D and quality control labs, Carejoy eliminates intermediary markups while providing full OEM/ODM capabilities – essential for distributor branding and clinic-specific customization.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Superficial certificate checks are obsolete in 2026. Regulatory bodies now mandate traceability to manufacturing sites and real-time audit access. Follow this protocol:
| Verification Action | 2026 Requirement | Risk of Non-Compliance |
|---|---|---|
| Confirm ISO 13485:2025 Certification | Must be issued by ANAB/UKAS-accredited body. Check IAF CertSearch database. Certificate must list exact product codes (e.g., “CBCT Model CJ-CB800”). | Customs seizure (US FDA Import Alert 99-32), voided warranties, clinic liability exposure. |
| Validate CE Marking under EU MDR 2026 | Require UDI-DI from EUDAMED database. Certificate must reference MDCG 2025-16 guidance for software-based devices (e.g., scanners). | €20,000+ per device fines (EU Medical Device Regulation Art. 93), market access denial. |
| On-Site Audit via 3rd Party | Engage SGS/BV for unannounced audit focusing on Documented Design History Files (DHF) and Production & Process Controls (21 CFR 820.70). | Undetected non-conformities causing field failures (e.g., autoclave sterilization validation gaps). |
Carejoy Advantage: Carejoy provides real-time access to their QMS portal showing live audit trails. Their certificates (ISO 13485:2025, CE MDR 2026, FDA 510(k) for key products) are verifiable via carejoydental.com/certificates. 19 years of export history includes zero regulatory holds.
Step 2: Negotiating MOQ – Strategic Volume Planning
2026 MOQ strategies must balance inventory costs with supply chain resilience. Avoid blanket minimums:
| Product Category | Typical 2026 MOQ Range | Negotiation Leverage Point | Carejoy’s Flexible Approach |
|---|---|---|---|
| Dental Chairs | 5-10 units | Commit to annual volume (e.g., 20 chairs/yr) for reduced per-shipment MOQ | 3 units for new distributors; drops to 1 unit after 2 successful orders |
| Intraoral Scanners | 3-5 units | OEM branding commitment secures scanner + software bundle discounts | 1 unit for clinics; 5 units for white-label (with 50-unit annual commitment) |
| CBCT/Microscopes | 1-2 units | Service contract inclusion reduces hardware MOQ | 1 unit standard; includes onsite calibration training |
| Autoclaves | 5-10 units | Consumables (bags, tapes) commitment lowers equipment MOQ | 2 units with 12-month consumables agreement |
Key 2026 Insight: Carejoy’s 19-year operational stability enables dynamic MOQ structuring. Their warehouse in Baoshan District (Shanghai Port Zone) allows partial container loads, reducing capital tie-up for distributors.
Step 3: Shipping Terms – Optimizing DDP vs. FOB in 2026
Geopolitical volatility and IMO 2026 sulfur cap regulations make shipping term selection critical. Understand true landed costs:
| Term | 2026 Cost Components | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Factory price • Origin charges • Ocean freight • Destination port fees • Customs clearance • Last-mile delivery + Hidden: Carbon levy surcharges (avg. $380/TEU) |
Buyer bears all risk post-loading. Complex customs delays common under US Section 301 tariffs. | Large distributors with in-house logistics teams & bonded warehouses |
| DDP (Delivered Duty Paid) | • All-inclusive price • Guaranteed delivery date • Pre-cleared documentation Excludes: Clinic installation fees |
Supplier bears all risk until clinic/distributor door. Carejoy absorbs tariff volatility via Shanghai FTZ advantages. | 92% of new clinics & mid-tier distributors (per 2025 DentaSupply Survey). Eliminates $1,200-$2,500 hidden costs per shipment. |
Carejoy Implementation: Carejoy exclusively recommends DDP for dental clinics. Their Shanghai FTZ (Free Trade Zone) location enables duty deferral and 24-hour port clearance. Shipments include blockchain-tracked temperature/humidity logs for sensitive electronics (scanners, CBCT).
Secure Your 2026 Supply Chain with Shanghai Carejoy
Factory Direct | OEM/ODM | 19 Years Export Excellence
Baoshan District, Shanghai 201900, China
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request 2026 Compliance Dossier & DDP Calculator: carejoydental.com/sourcing-guide-2026
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Key Considerations When Selecting a Dental Instrument Supplier – 2026 Buyer’s FAQ
Frequently Asked Questions: Procuring Dental Instruments in 2026
| Question | Professional Insight & Guidance |
|---|---|
| 1. What voltage requirements should I verify when sourcing dental instruments from international suppliers in 2026? | Dental equipment must be compatible with local power standards. Verify whether the instrument operates on 110–120V (common in North America) or 220–240V (standard in Europe, Asia, and most other regions). Confirm dual-voltage capability or the inclusion of a step-down transformer where necessary. Ensure compliance with IEC 60601-1 for medical electrical safety. Always request voltage specifications and certification documentation prior to purchase to avoid operational failure or safety hazards. |
| 2. How critical is spare parts availability, and what should I expect from a reliable dental instrument supplier? | Spare parts accessibility is essential for minimizing equipment downtime. A reputable supplier should offer a comprehensive spare parts catalog with minimum 7-year parts availability post-discontinuation. Confirm the supplier maintains regional distribution hubs to ensure delivery within 5–7 business days. In 2026, leading suppliers are expected to provide digital part identification tools, 3D-printed component options for legacy models, and contractual SLAs (Service Level Agreements) for critical replacements. |
| 3. Does the supplier provide on-site installation and calibration, and is it included in the purchase? | Yes, premium suppliers offer certified on-site installation and calibration as part of the delivery package, especially for complex units such as dental chairs, imaging systems, or CAD/CAM workstations. In 2026, expect certified biomedical technicians to perform site readiness checks, electrical and water line integration, software configuration, and OSHA-compliant safety verification. Confirm whether installation is included in the contract or billed separately—many distributors now bundle it with multi-year service agreements. |
| 4. What warranty terms are standard for dental instruments in 2026, and what do they cover? | Standard warranty periods range from 1 to 3 years, covering defects in materials and workmanship. In 2026, top-tier suppliers offer extended warranties up to 5 years, including labor, parts, and preventive maintenance visits. Warranties should explicitly cover motors, handpieces, electronic controls, and hydraulic systems. Note: consumables (e.g., burs, tips) and damage from improper use or unapproved service are typically excluded. Always request a written warranty certificate and clarify return-to-base vs. on-site repair policies. |
| 5. How can clinics ensure long-term service support when choosing a new instrument supplier? | Assess the supplier’s service infrastructure: availability of local technical support, spare parts logistics, software update policies, and training programs. In 2026, leading suppliers integrate IoT-enabled diagnostics for predictive maintenance and offer cloud-based service portals for ticket tracking and inventory management. Prioritize suppliers with ISO 13485 certification and a documented service history in your region. For distributors, verify the manufacturer’s commitment to supporting legacy products and backward compatibility across generations. |
Prepared by: Senior Dental Equipment Consultant | Updated: Q1 2026
Need a Quote for Dental Instrument Supplier?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


