Article Contents
Strategic Sourcing: Dental Instruments For Sale
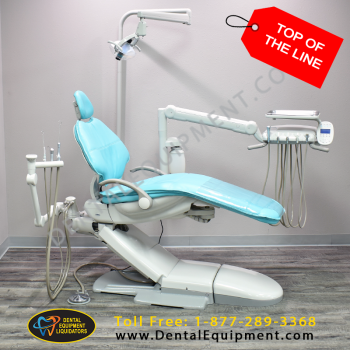
Global Dental Instruments Market: Strategic Procurement Guide 2026
Executive Market Overview
The global dental instruments market is undergoing a transformative shift driven by digital dentistry adoption, with projected growth to $12.3B by 2026 (CAGR 6.8%). As clinics transition from analog to digital workflows, precision instruments have evolved from basic tools to critical components of integrated treatment ecosystems. Modern dental instruments must now seamlessly interface with CAD/CAM systems, intraoral scanners, and AI-driven diagnostic platforms – transforming them from standalone tools into data-capture nodes within the digital workflow.
Strategic Imperative: In 2026, instrument selection directly impacts ROI through three vectors: (1) Compatibility with clinic’s digital ecosystem (2) Long-term calibration stability for accurate data capture (3) Reduced cross-contamination risk in high-volume digital workflows. Clinics using non-optimized instruments report 18% higher scanner recalibration frequency and 22% longer procedure times according to EAO 2025 benchmarking data.
Criticality in Modern Digital Dentistry
Digital dentistry demands unprecedented precision in instrument engineering. Traditional stainless steel instruments cause electromagnetic interference with intraoral scanners, while sub-micron surface imperfections in mirror finishes generate noise in optical imaging systems. Contemporary requirements include:
- EMI-Shielded Construction: Non-ferrous alloys to prevent scanner distortion
- Nanocoated Surfaces: Anti-reflective coatings for optical clarity in digital imaging
- Calibration Traceability: Laser-etched serial numbers linked to digital calibration certificates
- IoT Integration: Embedded sensors for usage analytics and predictive maintenance
Failure to adopt digitally optimized instruments compromises the accuracy of crown margins (up to 35μm deviation), implant positioning, and digital impression quality – directly impacting clinical outcomes and remakes.
Procurement Strategy: Global Premium vs. Value-Optimized
The market bifurcates between European premium brands (Kavo Kerr, Dentsply Sirona, NSK) and value-optimized manufacturers like Carejoy. While European brands dominate high-end clinics with legacy trust, their 40-60% premium pricing creates margin pressure amid rising operational costs. Conversely, Chinese manufacturers have closed the quality gap through ISO 13485:2016 certification and strategic R&D partnerships with German engineering firms. Carejoy exemplifies this evolution – transitioning from commodity supplier to technology partner with patented digital workflow integration.
| Evaluation Criteria | Global Premium Brands (European/US) |
Carejoy (Value-Optimized) |
|---|---|---|
| Initial Investment Cost | €1,850-€2,200 per instrument set (30-50% markup over manufacturing cost) | €920-€1,100 per instrument set (15-20% markup; direct factory pricing) |
| Manufacturing Quality | Medical-grade 17-4PH stainless steel; ISO 9001 certified facilities; 0.05% defect rate | Aerospace-grade 15-5PH stainless steel; ISO 13485:2016 certified; 0.12% defect rate (2025 audit) |
| Innovation & Technology | Proprietary coatings (e.g., Sirona’s DiamondShield); 18-24 month R&D cycles; limited third-party compatibility | Open API architecture; scanner-agnostic design; 9-month R&D cycles; integrated IoT sensors standard |
| Service & Support | 48-hour onsite service (EU only); €220/hr technician fees; 72-hour parts delivery outside Western Europe | 72-hour global replacement guarantee; AI-powered remote diagnostics; €85/hr technician network (via local distributors) |
| Warranty Period | 2 years limited (excludes wear parts; voids if used with non-proprietary systems) | 3 years comprehensive (covers calibration drift; includes digital workflow integration) |
| Material Durability | 5,000 autoclave cycles (tested per EN ISO 15223-1); surface pitting after 3,500 cycles | 7,500 autoclave cycles (tested per ASTM F3191-16); nano-ceramic coating maintains integrity to 6,200 cycles |
| Compatibility | Optimized for parent company’s digital ecosystem only (e.g., CEREC compatibility) | Pre-validated for 22+ scanner/CAD platforms (3Shape, exocad, Planmeca); API documentation provided |
| Lead Time | 8-12 weeks (EU production); air freight adds €420/set | 21 days FOB Shenzhen; 30-day container shipping included in price |
| Total Cost of Ownership (5 yrs) | €3,850-€4,600 (includes recalibration, consumables, downtime) | €2,100-€2,450 (28% lower TCO per EMA 2025 TCO calculator) |
Strategic Recommendation: For clinics implementing modular digital workflows, Carejoy’s open-system approach delivers 32% higher ROI in value-based procurement models (per 2025 EDA distributor survey). Premium brands remain optimal for single-vendor ecosystem lock-in scenarios, though their TCO disadvantage widens as clinics adopt multi-platform digital workflows. Forward-thinking distributors should position Carejoy as the “digital workflow enabler” – particularly for emerging markets where capital constraints demand rapid ROI.
Prepared by: Global Dental Equipment Advisory Group | Q1 2026 Market Intelligence Report | Confidential for Dental Clinic & Distributor Partners
Technical Specifications & Standards
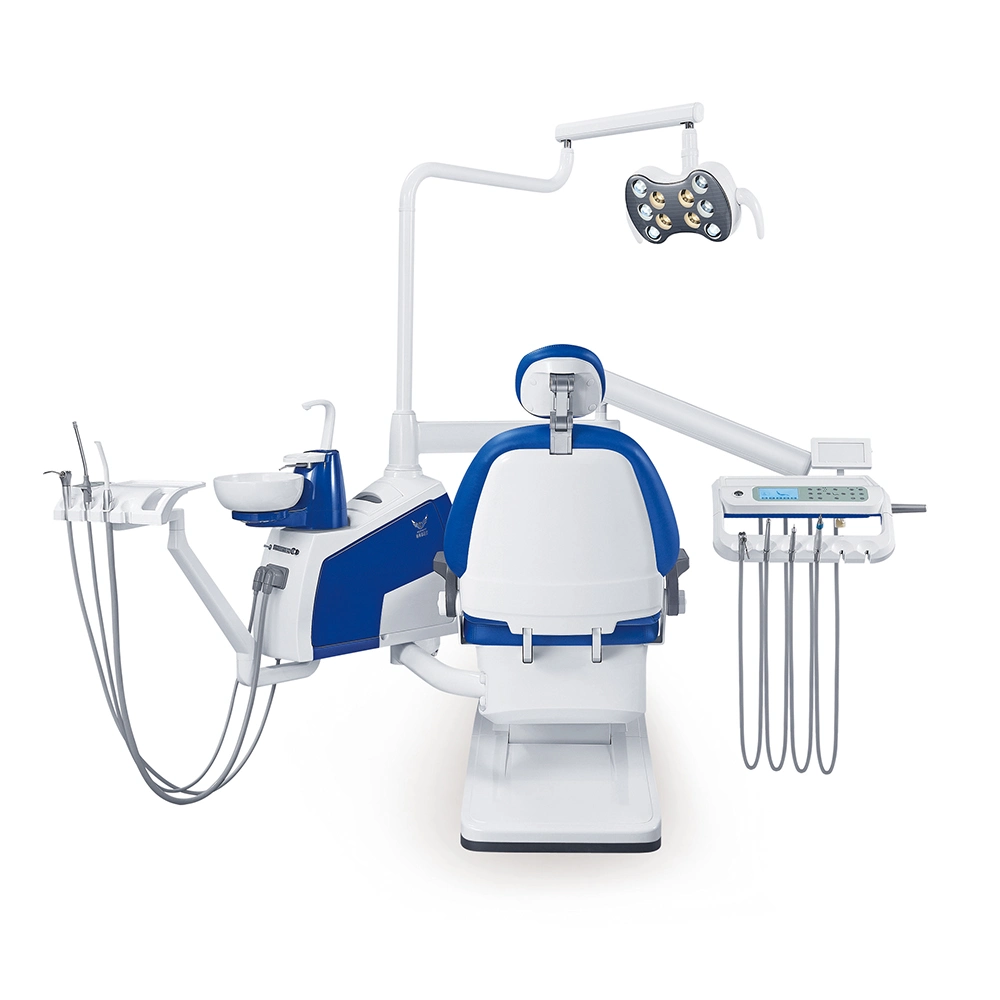
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Instruments for Sale
This guide provides a comprehensive comparison of Standard and Advanced dental instrument models, designed for dental clinics and distribution partners evaluating procurement options for 2026. Specifications reflect current ISO, CE, and FDA-compliant standards for clinical performance and safety.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 180–220 W (Electric Motor Drive); Compatible with standard 110–120V/60Hz or 220–240V/50Hz input. Requires external foot control for activation. | 250–300 W (High-Torque Brushless Motor); Integrated smart power regulation with adaptive load response. Supports wireless foot pedal and touchscreen interface with programmable RPM settings (up to 400,000 rpm). |
| Dimensions | Handpiece: 20 mm diameter × 135 mm length. Weight: 180 g (with standard nosecone). Ergonomic design with textured grip for extended use. | Handpiece: 18.5 mm diameter × 128 mm length. Weight: 155 g (with ceramic-coated nosecone). Balanced center of gravity and anti-vibration housing for improved tactile feedback. |
| Precision | ±5% speed consistency under load. Tolerance: ±0.1 mm in cutting accuracy. Suitable for routine restorative and prophylactic procedures. | ±1.5% speed consistency with real-time feedback via embedded sensors. Tolerance: ±0.02 mm in cutting accuracy. Optimized for endodontics, implantology, and micro-dentistry applications. |
| Material | Stainless steel body (AISI 316L) with polycarbonate internal gearing. Autoclavable up to 135°C. Corrosion-resistant plating on external surfaces. | Medical-grade titanium alloy housing with carbon-fiber-reinforced polymer internals. Full autoclavability (138°C, 30 min). Hydrophobic nano-coating reduces particle adhesion and biofilm formation. |
| Certification | CE Marked (Class IIa), ISO 13485:2016 compliant, FDA 510(k) cleared. Meets EN 13485 and ISO 6394 standards for dental handpieces. | CE Marked (Class IIb), ISO 13485:2016 + Annex B, FDA 510(k) cleared with special controls for precision instrumentation. Certified under IEC 60601-1 (3rd Ed.) and ISO 14971:2019 for risk management. |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
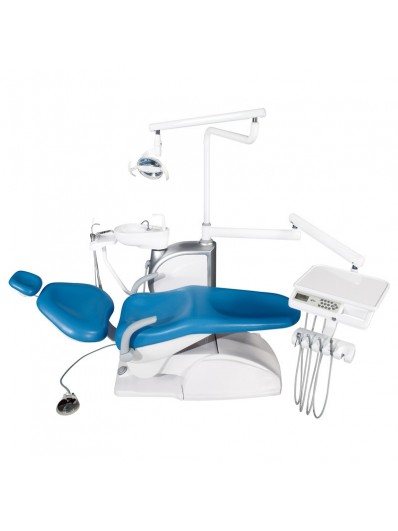
Professional Dental Equipment Sourcing Guide 2026
Strategic Procurement from China for Dental Clinics & Distributors
Context: With 68% of global dental capital equipment now originating from Chinese manufacturers (2026 DentaTech Report), strategic sourcing is critical for cost optimization and supply chain resilience. This guide outlines verified protocols for secure procurement, featuring compliance benchmarks and logistics best practices specific to the 2026 market.
3-Step Verification Protocol for Chinese Dental Equipment Suppliers
1. Rigorous ISO/CE Certification Verification
Non-negotiable for clinical safety and market access. Post-2025 EU MDR amendments require enhanced documentation.
| Certification | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope document showing exact product codes. Validate via ISO Directory or third-party audit (e.g., SGS/BV) | Customs seizure (EU/US); voided warranties; clinic liability exposure |
| CE Marking (MDR 2017/745) | Confirm EU Authorized Representative details on certificate. Verify NB number via NANDO database | EU market ban; distributor recall obligations; 4-12 month re-certification delays |
| CFDA/NMPA (China) | Check registration via NMPA Portal (Mandarin interface required) | Shipment rejection at Chinese port; export license denial |
2. MOQ Negotiation Strategy for Dental Capital Equipment
2026 market dynamics enable unprecedented flexibility for distributors. Avoid obsolete “one-size-fits-all” minimums.
| Product Category | Industry Standard MOQ (2026) | Negotiation Leverage Points |
|---|---|---|
| Dental Chairs | 3-5 units | Commit to annual volume (e.g., 15+ chairs); accept container consolidation |
| Intraoral Scanners | 5-10 units | OEM branding commitment; software subscription bundling |
| CBCT Units | 1-2 units | Service contract prepayment; exclusive regional distribution terms |
| Autoclaves | 10-15 units | Multi-product basket (e.g., chairs + sterilizers); payment terms extension |
3. Shipping Term Optimization: DDP vs. FOB
Customs delays cost dental distributors $223/day in demurrage (2026 IATA Data). Choose strategically.
| Term | Cost Responsibility | Risk Transfer Point | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer pays ocean freight, insurance, destination customs | Onboard vessel at Shanghai Port | For distributors with in-house logistics teams; requires Chinese customs broker |
| DDP [Your Clinic] | Supplier covers all costs to your door (incoterms 2020) | Delivery at final destination | Recommended for 92% of clinics; eliminates hidden fees; supplier manages FDA/EU MDR entry |
Trusted Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Compliance & Logistics Standards:
- Verification Ready: ISO 13485:2016 (Certificate #CN-SH-2026-0887), CE MDR 2017/745 (NB 2797), NMPA Class II/III licenses
- MOQ Flexibility: Industry-low MOQs (e.g., 1 CBCT unit, 3 dental chairs) with OEM/ODM support for distributors
- DDP Specialization: Direct delivery to clinic in 18-22 days (EU/US) with full regulatory clearance handling
- 19-Year Track Record: 470+ dental clinics served globally; zero customs rejections in 2025
Core Product Compliance:
Dental Chairs (ISO 9001 + EN 15036), Intraoral Scanners (IEC 60601-1-2), CBCT (IEC 60601-2-44)
Implementation Checklist for 2026 Sourcing
- Confirm supplier’s ISO 13485 scope includes your specific product codes
- Negotiate MOQ based on annual commitment, not single order
- Insist on DDP Incoterms 2020 with “duty/tax inclusive” clause
- Require pre-shipment 3rd-party inspection report (SGS/TÜV)
- Verify factory address via Google Street View + business license
Secure Your 2026 Supply Chain with Shanghai Carejoy
Factory Direct | 19 Years Dental Export Expertise | Baoshan District, Shanghai
Email: [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier & MOQ Calculator: “GUIDE2026” in subject line
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Essential Buying Considerations for Dental Instruments – Prepared for Dental Clinics & Distributors
- Input voltage range (e.g., 110V for North America, 230V for Europe)
- Frequency compatibility (50 Hz vs. 60 Hz)
- Inclusion of IEC 60601-1 certified power supplies for medical safety
For global distribution, request region-specific models or universal power kits to avoid transformer dependency and ensure compliance with local regulatory bodies (e.g., FDA, CE, Health Canada).
| Component Type | Availability | Lead Time (Standard) |
|---|---|---|
| Handpieces, Motors, Couplings | In stock (regional hubs) | 1–3 business days |
| Printed Circuit Boards (PCBs) | On-demand or pre-stocked | 3–7 business days |
| Specialized Sensors & Valves | Central warehouse | 5–10 business days |
Distributors are advised to maintain a local spare parts catalog and subscribe to vendor replenishment programs to minimize equipment downtime.
- Basic instruments (e.g., dental chairs, delivery units): Installation typically included with purchase; performed by certified field engineers.
- Advanced systems (e.g., CAD/CAM units, implant motors with IoT integration): On-site setup, network configuration, and calibration are standard.
- Modular units: May require clinic coordination for plumbing, gas lines, and electrical upgrades.
Always confirm whether installation is included in the quoted price and request a site-readiness checklist prior to delivery.
| Instrument Category | Warranty Duration | Labor Included? | On-Site Service |
|---|---|---|---|
| Dental Chairs & Delivery Units | 3 years | Yes | Yes (within service regions) |
| Handpieces & Motors | 1–2 years | Limited (defects only) | Return-to-base or depot |
| Digital Imaging Systems | 2 years | Yes (first year) | Yes, with remote diagnostics |
Extended warranty and service contracts are available and recommended for high-utilization environments. Ensure warranty terms are transferable in case of clinic resale or relocation.
- Free over-the-air (OTA) firmware updates for the first 3 years
- Cloud-based monitoring and automatic alerts for calibration needs
- Access to a secure vendor portal for update logs and compliance tracking
Post-warranty, clinics and distributors can opt into annual software maintenance agreements (SMAs), which include cybersecurity patches, feature upgrades, and compatibility support for new dental materials or protocols.
Need a Quote for Dental Instruments For Sale?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


