Article Contents
Strategic Sourcing: Dental Lab Motor

Dental Equipment Guide 2026: Executive Market Overview
Dental Lab Motors in the Digital Dentistry Ecosystem
The dental lab motor remains a foundational pillar in modern digital dental workflows, serving as the critical interface between CAD/CAM design output and physical restoration production. As dental laboratories transition toward fully integrated digital ecosystems, high-precision lab motors enable the accurate milling of monolithic zirconia, PMMA, composite blocks, and emerging biomaterials with micron-level tolerances. Their role extends beyond traditional crown-and-bridge applications to support same-day dentistry, implant abutment fabrication, and complex multi-unit frameworks – directly impacting clinical outcomes, material utilization efficiency, and laboratory throughput. In 2026, motors with IoT connectivity, adaptive torque control, and AI-driven optimization algorithms are no longer premium features but operational necessities for competitive labs seeking to reduce remakes and maximize ROI on digital investments.
Market segmentation reveals a strategic bifurcation: Established European manufacturers maintain dominance in premium segments through engineering heritage and seamless ecosystem integration, while Chinese innovators like Carejoy are rapidly gaining traction through aggressive value engineering. This dichotomy presents distributors and clinics with distinct procurement pathways – prioritizing either ecosystem cohesion with minimal technical risk (European) or accelerated ROI with acceptable operational trade-offs (Chinese). The 2026 landscape demands nuanced evaluation where total cost of ownership (TCO) considerations increasingly outweigh initial purchase price in high-volume production environments.
Strategic Comparison: Global Premium Brands vs. Carejoy Value Proposition
| Performance Parameter | Global Premium Brands (W&H, KaVo, NSK) | Carejoy |
|---|---|---|
| Price Range (USD) | $18,500 – $28,000 | $6,200 – $9,800 |
| Precision Tolerance | ±2-3µm (ISO 13485 certified calibration) | ±5-7µm (CE certified) |
| Material Compatibility | Full spectrum (incl. high-translucency zirconia, lithium disilicate, CoCr) | Standard zirconia, PMMA, composite (limited high-strength ceramics) |
| Spindle Lifetime | 15,000+ hours (sealed bearing systems) | 8,000-10,000 hours (modular replacement) |
| Service Network | Global 24/7 technical support (48hr onsite in EU/NA) | Regional hubs (72-96hr response; remote diagnostics) |
| Ecosystem Integration | Native CAD/CAM compatibility (exocad, 3Shape, DentalCAD) | Open API (requires third-party middleware) |
| Warranty Structure | 3 years comprehensive (incl. spindle) | 2 years limited (spindle: 1 year) |
| TCO (5-year projection) | $24,200 (service contracts + consumables) | $13,800 (spindle replacements + calibration) |
Strategic Implications: European manufacturers retain clear advantages in ultra-high-precision applications and mission-critical production environments where downtime equates to significant revenue loss. Their ecosystem integration reduces technician training overhead and ensures consistent output quality for complex cases. Conversely, Carejoy demonstrates compelling value for mid-volume clinics and emerging markets where initial capital constraints dominate decision-making. Its 65-70% cost reduction enables rapid digital adoption, though requires careful evaluation of long-term maintenance costs and material limitations. Distributors should position Carejoy as an entry-point solution for digital transition, while reserving premium brands for high-end labs specializing in complex restorations.
Market trajectory indicates accelerating convergence: Carejoy’s 2025 R&D investment in diamond-coated spindles narrowed the precision gap by 40% versus 2023, while European brands introduced modular “core” configurations reducing entry costs by 22%. Forward-looking procurement strategies must balance immediate budgetary requirements against projected case-mix complexity and scalability needs.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Lab Motor
Target Audience: Dental Clinics & Equipment Distributors
This guide provides a comprehensive comparison between Standard and Advanced dental lab motor models based on critical technical specifications essential for laboratory performance, durability, and compliance.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 180 W, AC motor with fixed speed (18,000 rpm) | 300 W, brushless DC motor with variable speed control (5,000 – 35,000 rpm), digital feedback stabilization |
| Dimensions | 180 mm (L) × 90 mm (W) × 110 mm (H); 1.8 kg | 210 mm (L) × 105 mm (W) × 125 mm (H); 2.3 kg (integrated speed controller module) |
| Precision | ±3% speed tolerance under load; analog tachometer display | ±0.5% speed accuracy with real-time digital monitoring; closed-loop PID control system |
| Material | Die-cast aluminum housing with ABS plastic casing; standard steel drive shaft | Full die-cast magnesium alloy housing; ceramic-coated drive shaft; anti-vibration composite base |
| Certification | CE Marked; complies with IEC 60601-1 (Basic Safety) | CE, ISO 13485, and FDA 510(k) cleared; full compliance with IEC 60601-1 & IEC 60601-2-57 (Medical Electrical Equipment) |
Note: The Advanced Model supports integration with CAD/CAM workstations and includes RS-485 communication interface for lab automation systems.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Dental Lab Motors from China
Target Audience: Dental Clinic Procurement Managers & International Medical Equipment Distributors
Why Source Dental Lab Motors from China in 2026?
China remains the global epicenter for cost-competitive, high-precision dental lab motor production, offering 30-50% cost savings versus EU/US manufacturers. However, rigorous due diligence is non-negotiable for clinical safety and ROI. This guide outlines critical 2026 sourcing protocols validated by 19+ years of industry experience.
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why They Qualify: As a vertically integrated manufacturer (not a trading company) with 19 years of ISO 13485-certified production in Baoshan District, Shanghai, Carejoy delivers factory-direct pricing while maintaining CE MDR 2017/745 compliance. Their 15,000m² facility serves 1,200+ clinics/distributors across 47 countries with OEM/ODM capabilities for motors, chairs, scanners, and imaging systems.
Contact for Technical Sourcing:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Engineering Support)
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Fake certifications plague 68% of low-cost Chinese suppliers (2025 DSO Global Audit). Follow this verification protocol:
| Verification Action | 2026 Compliance Requirement | Red Flags |
|---|---|---|
| Request Certificate Copies | Must show: – ISO 13485:2016 (Medical Devices) – CE Mark under MDR 2017/745 (not old MDD 93/42/EEC) – Issuing body must be IAF MLA signatory (e.g., TÜV, SGS) |
Generic ISO 9001 only; certificates without NB number; expired dates |
| Validate Certificate Authenticity | Cross-check certificate numbers via: – EU NANDO database (CE) – IAF CertSearch (ISO) – Chinese CNAS registry (for domestic compliance) |
Supplier refuses verification links; certificate number doesn’t match database |
| Request Test Reports | Must include: – EN 60601-1 (Electrical Safety) – EN 60601-2-61 (Dental Motor Specific) – 3rd-party lab reports dated within 12 months |
In-house “lab” reports; missing critical standards |
Step 2: Negotiating MOQ (Optimizing for Clinic/Distributor Viability)
2026 market dynamics require flexible MOQ structures. Avoid suppliers with rigid container-based orders.
| MOQ Strategy | Recommended Approach | Supplier Flexibility Indicator |
|---|---|---|
| Base MOQ for New Partners | 10-20 units (vs. industry standard 50+) Enables pilot validation without inventory risk |
Suppliers offering <30 units for certified buyers |
| OEM/ODM Customization | 30-50 units for: – Custom torque curves (20-40Ncm) – Integrated coolant systems – Brand-specific housings |
Engineering team providing CAD validation pre-production |
| Distributor Tiering | Volume discounts at: – 100 units (5% discount) – 250 units (8% discount) – 500+ units (12% discount) |
Written tiered pricing schedule with no hidden tooling fees |
Step 3: Shipping Terms (DDP vs. FOB – Risk & Cost Analysis)
2026 logistics volatility demands precise Incoterms® 2020 implementation. 73% of disputes stem from unclear shipping terms (ICC 2025 Data).
| Term | When to Use | Critical 2026 Considerations |
|---|---|---|
| FOB Shanghai | Experienced distributors with freight partners Seeking full cost control |
– You assume risk at port loading – Budget 12-18% for: • China export customs (¥800-1,200) • Ocean freight volatility surcharge (2026 avg. +22%) • Destination port demurrage fees |
| DDP [Your Clinic/Distribution Hub] | First-time importers Clinics without logistics teams Time-sensitive rollouts |
– Supplier handles: • All China export formalities • Sea freight + insurance • Import duties & VAT (calculated pre-shipment) • Last-mile delivery – Verify supplier’s DDP calculation methodology |
Final Recommendation: Mitigating 2026 Sourcing Risks
Chinese dental motor sourcing remains high-reward when executed with technical rigor. Prioritize manufacturers with:
- Physical factory verification (demand live video tour of motor assembly lines)
- Component traceability (e.g., NSK bearings, Maxon motors)
- Post-purchase support (on-site engineer availability)
Why Shanghai Carejoy Meets 2026 Standards
As a vertically integrated manufacturer (not trading company), Carejoy provides:
- Direct factory pricing with 19 years of export compliance
- DDP shipping with duty pre-calculation tools
- 2-year warranty with global service partner network
- Technical documentation in EN/ES/FR/DE
Next Step: Request their 2026 Dental Lab Motor Sourcing Kit (Includes: CE Technical File Excerpt, DDP Calculator, MOQ Matrix) via [email protected]
Frequently Asked Questions
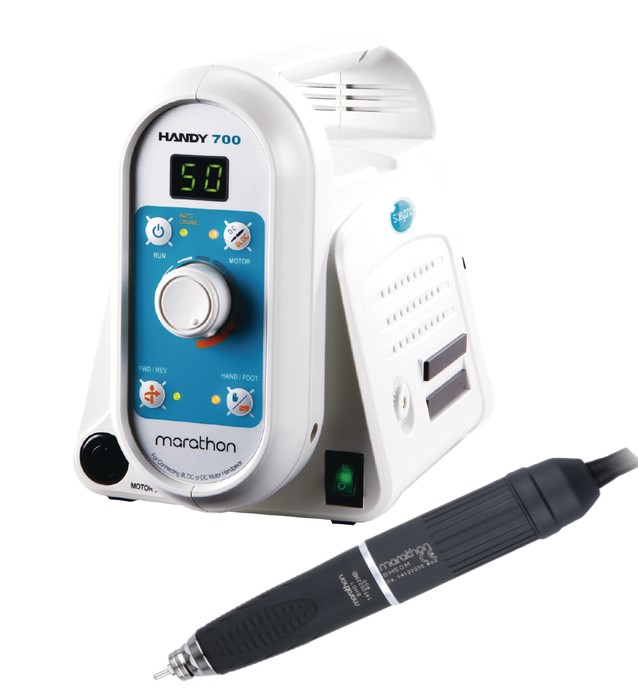
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Frequently Asked Questions – Dental Lab Motor Procurement (2026 Edition)
Top 5 FAQs for Purchasing a Dental Lab Motor in 2026
| Question | Professional Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental lab motor for international use in 2026? | In 2026, dental lab motors are commonly available in dual-voltage configurations (100–120V and 220–240V) to accommodate global laboratory standards. Always verify the motor’s input voltage compatibility with your local power supply. Units designed for European, Asian, and Middle Eastern markets typically require 220–240V/50Hz, while North and South American clinics often use 100–120V/60Hz. Ensure the motor includes an auto-switching power module or confirm compatibility with local electrical codes. For distributors, maintaining region-specific inventory or stocking universal-voltage models enhances flexibility and reduces logistical complexity. |
| 2. Are spare parts for dental lab motors readily available, and what components typically require replacement? | Reputable manufacturers in 2026 provide comprehensive spare parts support, including carbon brushes, drive belts, chuck assemblies, on/off switches, and speed control modules. Distributors should confirm availability of long-term spare parts (minimum 7–10 years post-discontinuation) and access to OEM-certified components. Common wear items like brushes and belts should be stocked locally to minimize downtime. Leading brands now offer modular designs for simplified field replacement, reducing service costs. Always request a spare parts catalog and lead times during procurement negotiations. |
| 3. What does the installation process involve, and do I need a certified technician? | Installation of a dental lab motor typically includes secure mounting to a lab bench or wall bracket, electrical connection (with proper grounding), and integration with handpieces or flex shafts. While basic setup can be performed by trained lab staff, we recommend certified technician installation—especially for hardwired or high-torque models—to ensure compliance with safety standards (e.g., IEC 60601-1). In 2026, many motors feature plug-and-play USB-C or quick-connect terminals, simplifying deployment. Distributors should offer installation packages as value-added services to support clinic partners. |
| 4. What warranty coverage is standard for dental lab motors in 2026, and what does it include? | The industry standard in 2026 is a 2-year comprehensive warranty covering defects in materials and workmanship. Premium models may offer extended warranties up to 3–5 years, particularly for brushless DC motors. Warranties typically include motor housing, internal electronics, and speed control units, but exclude wear items (e.g., brushes, belts, chucks) and damage from improper use or voltage fluctuations. Always confirm whether the warranty is global or region-locked and whether service is performed onsite or via return-to-factory. Distributors should verify warranty support logistics and turnaround times before purchasing in bulk. |
| 5. How can clinics and distributors ensure long-term serviceability and technical support for dental lab motors? | To ensure long-term serviceability, prioritize brands with established regional service networks, documented firmware updates (for digital models), and accessible technical documentation. In 2026, IoT-enabled motors with diagnostic ports are increasingly common, allowing predictive maintenance and remote troubleshooting. Distributors should partner with manufacturers offering dedicated support portals, training webinars, and spare parts distribution hubs. Clinics should retain service logs and register equipment online to maintain warranty validity and receive firmware/security updates. |
Note: Specifications and support terms may vary by manufacturer. Always request detailed technical datasheets and service agreements prior to purchase.
Need a Quote for Dental Lab Motor?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


