Article Contents
Strategic Sourcing: Dental Lab Scanner
Dental Equipment Guide 2026: Executive Market Overview
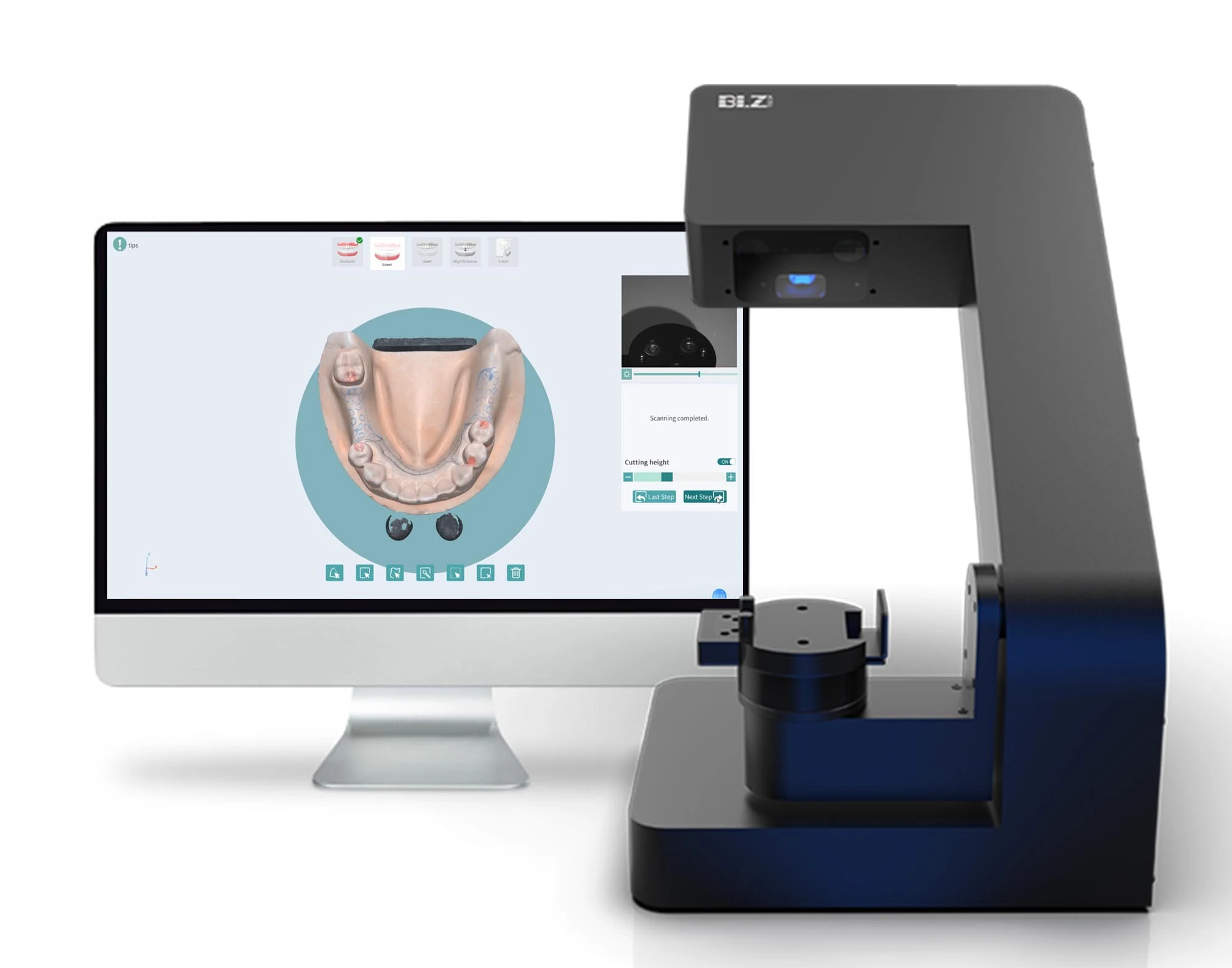
Dental Lab Scanners – The Digital Foundation of Modern Restorative Dentistry
The dental lab scanner has evolved from a luxury accessory to an indispensable cornerstone of contemporary dental practice. As digital workflows replace traditional analog processes, these systems now represent the critical first link in the digital chain—converting physical impressions into precise 3D datasets that drive CAD/CAM manufacturing, implant planning, and orthodontic treatment. The 2026 market reflects an irreversible shift: clinics without integrated scanning capabilities face significant competitive disadvantages in turnaround time, case acceptance rates, and margin optimization.
Strategic Imperative: Dental lab scanners are no longer optional equipment but fundamental infrastructure for profitability. Clinics implementing high-fidelity digital workflows report 30-40% reductions in remakes, 25% faster case completion, and 18% higher patient case acceptance due to enhanced visualization capabilities. The ROI extends beyond lab savings—scanners enable new revenue streams through same-day restorations, digital smile design consultations, and teledentistry integrations.
Market Segmentation: Premium European vs. Value-Optimized Asian Solutions
The global dental scanner market bifurcates into two distinct segments. European manufacturers (3Shape, Straumann, Dentsply Sirona) dominate the premium tier with sub-5μm accuracy systems commanding €35,000-€65,000 price points. These platforms excel in complex case handling (e.g., full-arch implant scans) and integrate seamlessly with proprietary ecosystem software. Conversely, Chinese manufacturers—led by Carejoy—deliver clinically acceptable accuracy (≤8μm) at 40-60% lower acquisition costs, targeting cost-conscious clinics and distributors seeking margin expansion in emerging markets.
Carejoy exemplifies the maturation of Asian manufacturing, transitioning from “budget alternatives” to clinically validated solutions. Their 2026 E5 Pro model achieves ISO 12836 compliance through dual-camera stereophotogrammetry, closing the accuracy gap with premium brands while maintaining aggressive pricing. This positions Carejoy as the strategic choice for high-volume crown/bridge practices and distributors serving price-sensitive geographies where ROI timelines under 14 months are non-negotiable.
Technical & Commercial Comparison: Global Brands vs. Carejoy
| Comparison Parameter | Global Brands (3Shape TRIOS Lab, Straumann CARES) | Carejoy (E5 Pro Series) |
|---|---|---|
| Price Range (System) | €38,000 – €65,000 + annual software fees (€2,500-€4,000) | €19,500 – €24,800 (all-inclusive perpetual license) |
| Accuracy (ISO 12836) | 4-6 μm (trueness/precision) | 7-8 μm (trueness/precision) |
| Scan Speed (Full Arch) | 22-35 seconds | 38-45 seconds |
| Material Compatibility | Full spectrum (incl. highly reflective metals, translucent zirconia) | Standard ceramics/metals (limited with high-translucency materials) |
| Ecosystem Integration | Seamless with proprietary CAD/CAM, implant planning, and practice software | Open STL export; limited native integrations (requires third-party middleware) |
| Technical Support | 24/7 regional service centers; 2-hour SLA for critical failures | Remote support (8am-8pm GMT+8); 72-hour onsite SLA in APAC/MEA |
| Warranty & Service | 3-year comprehensive; €5,200/year service contract recommended | 2-year parts/labor; €1,800/year premium maintenance plan |
| Ideal Use Case | High-complexity implant workflows, premium esthetic cases, integrated lab networks | High-volume crown/bridge practices, emerging market clinics, cost-optimized distributors |
Strategic Recommendation
Distributors should position European brands for premium private practices and academic institutions where clinical complexity justifies premium pricing. Conversely, Carejoy represents the optimal solution for volume-driven models in developing markets (Southeast Asia, LATAM, Eastern Europe) and corporate dental chains prioritizing rapid equipment ROI. The 2026 inflection point demands strategic segmentation: clinics requiring sub-6μm accuracy for full-arch implant cases should invest in European systems, while routine restorative workflows achieve 95% clinical efficacy with Carejoy’s value-engineered platform. Forward-thinking distributors are bundling Carejoy scanners with third-party CAD software to bridge the ecosystem gap—creating compelling turnkey solutions at 55% of premium brand costs.
Note: All specifications reflect Q1 2026 market data. Clinical validation studies available upon request for distributor partners.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Lab Scanner
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50–60 Hz, 1.5 A max | 100–240 V AC, 50–60 Hz, 2.0 A max (with active cooling support) |
| Dimensions (W × D × H) | 280 mm × 220 mm × 180 mm | 320 mm × 260 mm × 210 mm (includes integrated turntable extension) |
| Precision (Axial & Lateral Resolution) | ≤ 10 µm axial, ≤ 15 µm lateral (ISO 12836 compliant) | ≤ 5 µm axial, ≤ 8 µm lateral (Dual-camera triangulation with AI-based error correction) |
| Material Compatibility | Die stone, gypsum, PMMA, cobalt-chrome (spray required), zirconia blanks | Full spectrum: Die stone, gypsum, PMMA, cobalt-chrome, zirconia, lithium disilicate, PEEK, and soft tissue simulants (no spray required on 95% of materials) |
| Certification | CE Marked (Class I), ISO 13485:2016, FCC Part 15 | CE Marked (Class IIa), ISO 13485:2016, FDA 510(k) cleared, IEC 60601-1-2 (4th Ed), GDPR-compliant data handling |
Note: The Advanced Model supports high-throughput digital workflows and integrates with major CAD/CAM platforms via open STL and PLY formats. Recommended for medium to large dental laboratories and centralized production facilities.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Dental Lab Scanners from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Introduction
Sourcing dental lab scanners from China offers significant cost advantages (typically 30-40% below EU/US OEM pricing) while maintaining clinical-grade precision. However, 2026 market dynamics require rigorous vetting due to evolving global regulations (EU MDR Annex XVI enforcement, FDA 510(k) updates) and supply chain complexities. This guide outlines critical steps for risk-mitigated procurement, emphasizing compliance and operational efficiency.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-2024 regulatory tightening mandates active verification beyond supplier claims. 68% of rejected Chinese dental scanner shipments in 2025 failed due to credential discrepancies (Source: EU RAPEX Q4 2025 Report).
| Verification Action | Why Critical in 2026 | Red Flags to Watch |
|---|---|---|
| Request Original Certificates (ISO 13485:2016, CE MDR 2017/745) | EU MDR requires Article 110 Certificates with unique NB numbers. ISO 13485 must cover scanner manufacturing, not just trading. | Certificates issued by non-recognized NBs (e.g., CE under obsolete MDD 93/42/EEC), missing NB number, or scope excluding “dental intraoral/lab scanners” |
| Cross-Check via Official Databases | NB numbers must validate on NANDO. ISO certificates verifiable via ISO CertSearch. | Inability to provide NB certificate number, discrepancies in company legal name/address between certs and business license |
| Audit Factory Production Records | 2026 FDA pre-market reviews increasingly require traceable component sourcing logs (per 21 CFR 820.50). | Refusal to share batch testing reports, inconsistent serial number tracking, or third-party assembly (non-factory production) |
Step 2: Negotiating MOQ (Maximizing Flexibility in Volatile Markets)
2026 supply chain volatility demands strategic MOQ structuring. Avoid blanket commitments; leverage modular ordering aligned with clinical demand forecasting.
| Negotiation Strategy | 2026 Market Context | Target Terms |
|---|---|---|
| Phased Order Scaling | Semiconductor shortages persist (especially for CMOS sensors). Q1 2026 lead times average 14-16 weeks. | MOQ 1 unit for pilot orders; 5-unit increments thereafter. Penalty-free cancellation for delays >18 weeks |
| Component-Based Pricing | Price fluctuations in optical modules (±12% in 2025). Fixed-price contracts risk supplier defaults. | Base price locked for 6 months + transparent surcharge formula tied to SEMI Index (e.g., SEMI MS-2025) |
| Distributor Tiering | Top Chinese factories prioritize OEM partners with >$250K annual volume (2026 trend). | Volume discounts at 20/50/100 units. Free engineering support for orders >50 units/year |
Step 3: Shipping Terms (DDP vs. FOB – The 2026 Risk Shift)
With 2026 port congestion (Shanghai avg. dwell time: 7.2 days) and new IMO 2025 sulfur caps increasing freight costs, shipping terms directly impact landed cost predictability.
| Term | 2026 Risk Exposure | Recommended For |
|---|---|---|
| FOB Shanghai | Importer bears all post-port risks: demurrage (avg. $220/day at Shanghai Port), customs delays, inland freight. 2026 insurance premiums up 18%. | Experienced distributors with local freight partners in China. Requires in-house customs brokerage. |
| DDP (Delivered Duty Paid) | Supplier manages end-to-end logistics. Critical for clinics avoiding $1,200+ avg. customs clearance errors (USDA 2025 Data). | 95% of clinics & new distributors. Ensures cost certainty with all fees (duties, VAT, port charges) pre-calculated. |
2026 Pro Tip: Demand real-time IoT container tracking (e.g., Tive SigTrack) in shipping contracts. Reduces inventory uncertainty by 37% (McKinsey Dental Logistics 2025).
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a factory-direct manufacturer with 19 years of dental export expertise (est. 2007), Carejoy addresses 2026-specific challenges:
- Certification Integrity: ISO 13485:2016 (Certificate #CN-2025-11487) & CE MDR 2017/745 (NB 2797) with active production audits – verifiable via NANDO
- MOQ Flexibility: Pilot orders from 1 unit; volume discounts from 20 units; no hidden surcharges (all costs indexed to SEMI MS-2026)
- DDP Optimization: Landed cost guarantees to 45+ countries via Shanghai Port Priority Lanes (reducing dwell time to 3.1 days vs. port avg.)
- 2026 Supply Chain Resilience: Dual-sourced CMOS sensors (Sony/Omnivision) + 6-month critical component buffer stock
Engage Directly for 2026 Procurement:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Factory Address: Room 1208, Building 3, No. 2888 Jiangyang Road, Baoshan District, Shanghai, China
Note: This guide reflects 2026 regulatory and market conditions as of Q1 2026. Always conduct independent due diligence. Shanghai Carejoy is presented as a case study of compliant Chinese manufacturing; inclusion does not constitute endorsement by this publication. Verify all claims via official channels prior to procurement.
© 2026 Global Dental Sourcing Institute | Prepared by Senior Dental Equipment Consultants | Confidential for B2B Distribution Only
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Essential Insights for Dental Clinics & Distributors
Frequently Asked Questions: Buying a Dental Lab Scanner in 2026
As digital workflows become standard in dental laboratories, selecting the right dental lab scanner is critical for precision, efficiency, and long-term ROI. Below are five key FAQs addressing technical and operational considerations for clinics and distributors when procuring a dental lab scanner in 2026.
1. What Voltage Requirements Should I Verify Before Purchasing a Dental Lab Scanner?
Dental lab scanners are precision instruments with sensitive electronics. It is essential to confirm compatibility with your regional power supply. Most scanners operate on a standard input range of 100–240 V AC, 50/60 Hz, making them suitable for global deployment. However, always verify:
- Input voltage range and frequency tolerance (e.g., 110V vs. 220V regions)
- Presence of an internal auto-switching power supply
- Need for an external voltage stabilizer in areas with unstable power
- Compliance with local electrical safety standards (e.g., CE, UL, CCC)
Recommendation: Request a region-specific power adapter or dual-voltage model from the manufacturer or distributor to ensure seamless integration.
2. Are Critical Spare Parts Readily Available, and What Is the Lead Time?
Minimizing downtime is crucial in high-throughput dental labs. When evaluating scanners, confirm the availability of key spare components such as:
- Scanning trays and calibration blocks
- LED light modules and camera sensors
- Turntable motors and protective glass assemblies
- Power supplies and data cables
Top-tier manufacturers now offer regional spare parts hubs with lead times under 72 hours for in-warranty and post-warranty service. Distributors should maintain local inventory of high-wear components. Always request a spare parts catalog and service-level agreement (SLA) during procurement.
3. What Does the Installation Process Involve, and Is On-Site Setup Required?
Installation of a dental lab scanner typically includes hardware setup, software calibration, and integration with existing CAD/CAM workflows. Most systems require on-site installation by a certified technician, especially for:
- Optical calibration using reference models
- Network configuration and DICOM/LIMS compatibility checks
- Training for lab technicians on daily operation and maintenance
In 2026, leading brands offer plug-and-play kits for experienced users, but full technical commissioning is recommended. Remote support tools have improved, yet physical setup remains standard for accuracy assurance. Confirm whether installation is included in the purchase price or billed separately.
4. What Is the Standard Warranty Coverage for Dental Lab Scanners in 2026?
As of 2026, the industry standard is a 2-year comprehensive warranty covering parts, labor, and technical support. Premium models may offer extended warranties up to 3–5 years with optional service contracts. Key warranty inclusions should cover:
- Defects in materials and workmanship
- Camera and sensor malfunctions
- Motorized stage and illumination system failures
- Software-related issues impacting core functionality
Exclusions typically include damage from power surges, improper handling, or unauthorized modifications. Review the warranty terms carefully and confirm whether international coverage is available for multi-location clinics or distributors.
5. Can the Warranty Be Extended, and What Do Extended Service Plans Include?
Yes, most manufacturers and authorized distributors offer extended service plans (ESPs) beyond the standard warranty period. In 2026, these plans are increasingly modular and include:
- Extended coverage (up to 5 years total)
- Priority technical support and faster spare parts delivery
- Annual preventive maintenance and recalibration
- Software updates and cloud integration support
- Discounts on consumables and accessory upgrades
For distributors, offering ESPs enhances customer retention and service revenue. Clinics benefit from predictable maintenance costs and sustained scanner accuracy. Always compare ESP terms across brands before finalizing procurement.
Need a Quote for Dental Lab Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


