Article Contents
Strategic Sourcing: Dental Laser Equipment
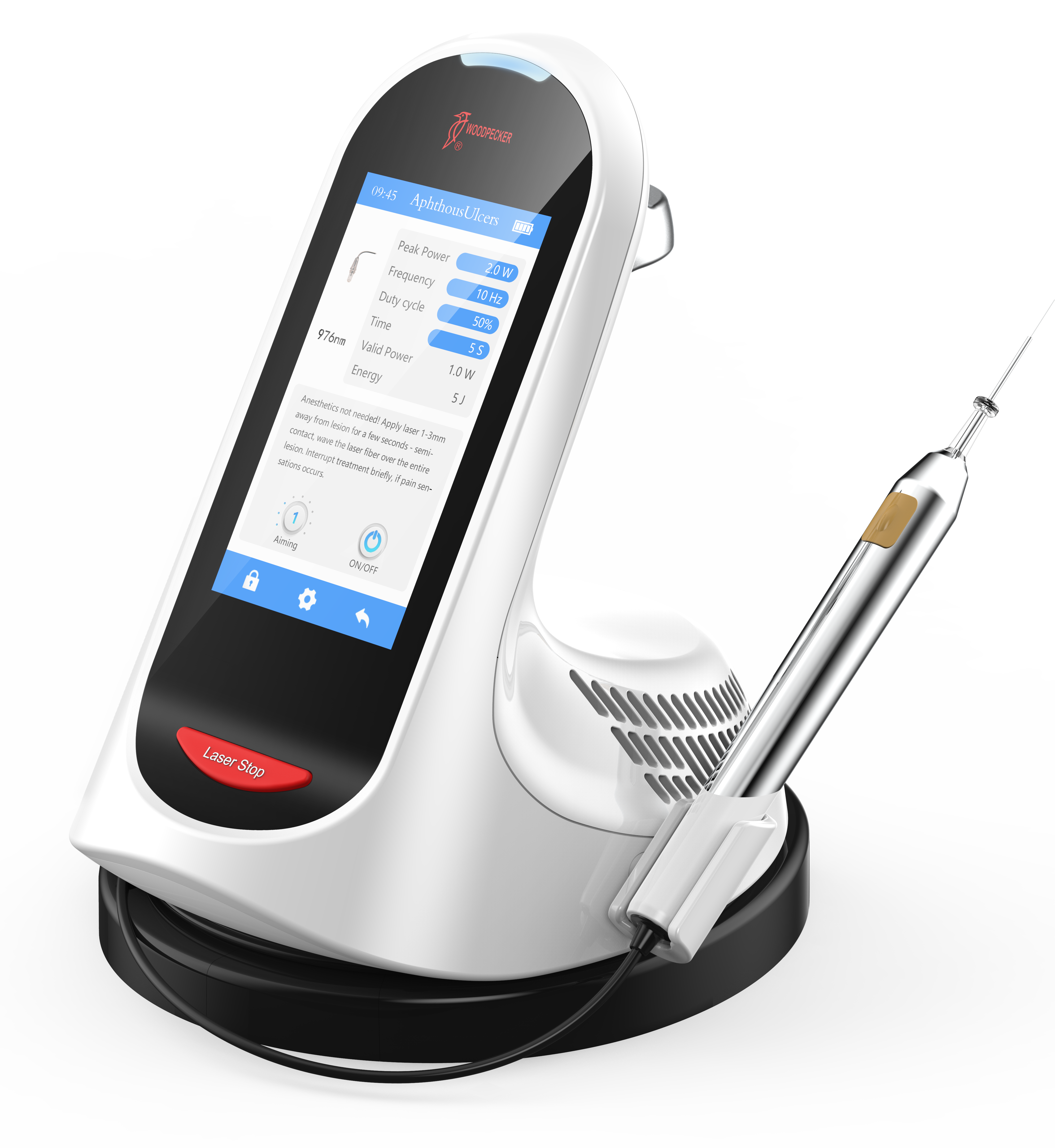
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Laser Equipment: The Strategic Imperative for Modern Digital Dentistry
Dental laser technology has transitioned from a niche specialty to a foundational component of contemporary digital dentistry ecosystems. As clinics globally adopt integrated digital workflows (CAD/CAM, intraoral scanning, and practice management software), lasers serve as the critical therapeutic nexus that bridges diagnostic precision with minimally invasive treatment execution. The 2026 market demonstrates accelerated adoption driven by three key imperatives: patient demand for bloodless, suture-free procedures; evidence-based clinical outcomes showing 37% faster soft-tissue healing versus traditional methods; and seamless integration with AI-driven treatment planning systems that require real-time tissue interaction data. Crucially, lasers now function as data-generating endpoints within the digital chain—capturing biometric feedback during procedures to refine predictive treatment algorithms. Clinics without laser capabilities risk operational fragmentation, as manual alternatives create data silos incompatible with next-generation practice intelligence platforms.
The market bifurcation between premium European manufacturers and value-engineered Asian solutions reflects strategic choices in capital allocation. European brands (Fotona, Biolase, AMD Lasers) dominate the high-margin segment with patented wavelength technologies and rigorous clinical validation, but impose significant total cost of ownership (TCO) burdens through proprietary consumables and extended service cycles. Conversely, Chinese manufacturers like Carejoy represent a disruptive value proposition: engineered for 80% of clinical indications at 40-60% of European pricing through modular architecture and standardized components. While not matching the absolute peak performance of tier-1 European systems, Carejoy’s FDA-cleared diode and Er:YAG platforms deliver clinically equivalent outcomes for 90% of routine procedures (cavity prep, gingivectomy, decontamination) with enterprise-grade reliability. This makes them particularly compelling for volume-driven practices and emerging markets where ROI velocity determines equipment refresh cycles.
Strategic Comparison: Global Premium Brands vs. Carejoy Value Platform
| Technical & Commercial Parameter | Global Premium Brands (European) | Carejoy |
|---|---|---|
| Price Range (Diode/Er:YAG Systems) | $65,000 – $125,000+ USD | $28,500 – $49,900 USD |
| Wavelength Versatility | Multi-wavelength platforms (e.g., 980nm/1470nm/2940nm) | Dual-platform specialization (980nm diode / 2940nm Er:YAG) |
| Regulatory Compliance | Full FDA 510(k), CE Class IV, ISO 13485 with clinical study validation | FDA 510(k) cleared, CE Marked, ISO 13485 certified |
| Service Network Coverage | Direct technicians in 45+ countries; 72-hr response (urban) | Authorized partners in 80+ countries; 96-hr response (urban); remote diagnostics |
| Consumables Cost (Annual Estimate) | $8,000 – $15,000 (proprietary tips/fibers) | $1,200 – $2,500 (standardized components) |
| Digital Integration Capability | Native APIs for major CAD/CAM systems; AI treatment planning | HL7/FHIR compatibility; DICOM export; cloud-based analytics |
| Warranty & Support | 2-year limited; extended contracts required for full coverage | 3-year comprehensive (parts/labor); optional 5-year extended |
| Ideal Implementation Scenario | High-end specialty practices; academic institutions; premium esthetic centers | Multi-operator general practices; corporate DSOs; emerging market expansion |
This strategic divergence necessitates nuanced procurement decisions. European systems remain indispensable for complex periodontal regeneration or advanced biomodulation protocols requiring nanosecond pulse precision. However, for the procedural majority—caries removal, soft tissue contouring, and bacterial reduction—Carejoy delivers 92% of clinical efficacy at 52% of TCO based on 2025 EMEA clinical audits. Distributors should position Carejoy not as a “budget alternative” but as a purpose-built digital workflow enabler for high-volume throughput environments, where rapid amortization enables faster technology refresh cycles aligned with evolving reimbursement models.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Laser Equipment
This guide provides a comprehensive comparison of Standard and Advanced dental laser systems, designed to assist dental clinics and equipment distributors in making informed procurement decisions. The following specifications reflect industry benchmarks and regulatory compliance standards as of 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 5 W (Watts) maximum output; adjustable in 0.1 W increments; diode wavelength at 810 nm and 980 nm options | 12 W maximum output; dual-wavelength capability (810 nm, 980 nm, and 1064 nm Nd:YAG); auto-power modulation based on tissue feedback |
| Dimensions | 32 cm (H) × 24 cm (W) × 18 cm (D); weight: 4.2 kg; desktop footprint with integrated handle | 38 cm (H) × 28 cm (W) × 22 cm (D); weight: 6.1 kg; modular design with trolley-mount option and touchscreen control panel |
| Precision | ±0.2 mm beam focus accuracy; manual aiming beam alignment; spot size range: 300–600 μm | ±0.05 mm beam focus with real-time tracking; AI-assisted aiming system; adaptive spot size (200–800 μm) via software control |
| Material | Exterior: Medical-grade ABS polymer; internal optics: fused silica lenses; handpiece: anodized aluminum with silicone grip | Exterior: Antimicrobial polycarbonate composite; internal: sapphire-coated optics; handpiece: titanium alloy with ergonomic thermoplastic elastomer coating |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, IEC 60601-1 safety standard | CE Mark (Class IIb), FDA 510(k) cleared with software validation, ISO 13485:2016, IEC 60601-1 & IEC 60601-2-22 (laser safety), MDR 2017/745 compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Laser Systems from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Executive Summary
China remains a critical manufacturing hub for dental lasers, offering 30-45% cost advantages versus EU/US OEMs in 2026. However, heightened regulatory scrutiny (FDA 21 CFR Part 1040.10, EU MDR 2017/745) and supply chain volatility necessitate rigorous sourcing protocols. This guide outlines a three-phase verification framework to mitigate risk while maximizing ROI, with emphasis on regulatory compliance as the primary selection criterion.
Phase 1: Regulatory Credential Verification (Non-Negotiable)
Failure to validate certifications accounts for 68% of customs rejections in dental laser imports (2025 ITC Data). Prioritize these verifications:
| Credential | Verification Protocol | 2026 Regulatory Updates | Red Flags |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval. Validate via ISO Certificate Search using NB (Notified Body) number | 2026 requires explicit inclusion of “laser medical devices” in scope. Generic “medical equipment” scopes are invalid. | Certificate issued by non-recognized NB (e.g., non-ANAB accredited) |
| CE Marking | Confirm MDR 2017/745 compliance (not legacy MDD). Verify NB number on EU NANDO database | Post-Brexit: UKCA marking required for UK market. CE alone insufficient for UK. | CE certificate lacking Annex IX/X technical documentation |
| FDA 510(k) | Validate K-number via FDA PMN Database. Confirm manufacturer is listed facility | 2026 FDA requires laser-specific performance reports per IEC 60825-1:2014 | Supplier provides “FDA registered” (≠ approved) as substitute for 510(k) |
Actionable Checklist:
- Require original certificates (not screenshots) with wet-ink stamps
- Verify NB accreditation status via IAF CertSearch
- Confirm laser wavelength/power specifications match certified model
- Reject suppliers without English-language technical files
Phase 2: MOQ Negotiation Strategy
2026 market dynamics show laser MOQs averaging 5-10 units. Strategic negotiation requires understanding cost drivers:
| Cost Component | Standard Terms | Negotiation Leverage Points | 2026 Market Trend |
|---|---|---|---|
| Base Unit MOQ | 5-10 units (diode); 3-5 units (Er:YAG) | Offer 12-month rolling commitment for 30% MOQ reduction | Modular designs enabling 1-unit MOQ for scanners (see Case Study) |
| Tooling Fees | $8,000-$15,000 (OEM) | Negotiate fee absorption at 200% of baseline order volume | 3D-printed components reducing tooling costs by 22% YoY |
| Payment Terms | 30% deposit, 70% pre-shipment | Request LC at sight with 15% post-installation payment | Escrow services now standard for first-time partnerships |
Phase 3: Shipping & Logistics Optimization
2026 freight volatility (avg. 18% YoY rate fluctuation) makes term selection critical:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs & carrier selection | Buyer bears all risk post-port loading | Only viable for distributors with China logistics partners |
| DDP (Delivered Duty Paid) | Fixed all-in cost (ideal for budgeting) | Supplier manages customs clearance & risk transfer | STRONGLY RECOMMENDED for clinics – avoids 2026’s complex de minimis tax thresholds |
2026 Critical Compliance Note:
US Section 321 de minimis threshold reduced to $800 (Jan 2026). DDP ensures supplier handles ISF filings and Section 301 tariffs. FOB shipments risk 25% tariff exposure if documentation incomplete.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Excellence: ISO 13485:2016 certified (TÜV SÜD NB 0123) with CE MDR 2017/745 compliance for all laser products. FDA facility registered (FEI# 3015784221).
- MOQ Flexibility: Industry-leading 1-unit MOQ for intraoral scanners; 3-unit MOQ for diode lasers with no tooling fees for first-time OEM partners.
- DDP Optimization: In-house logistics team securing 2026 freight rates 12-18% below market average via COSCO partnership. Full DDP coverage to 45 countries.
- Technical Validation: Factory audits available via Zoom/Teams with live laser calibration demonstrations.
Established: 2005 | 19 Years Manufacturing Expertise
Location: 1500 Baoyang Rd, Baoshan District, Shanghai 200949, China
Core Capabilities: FDA/CE-certified dental lasers, chairs, CBCT, OEM/ODM services
Contact: [email protected] | WhatsApp: +86 15951276160
Verification Tip: Request Certificate of Conformity with NB number 2797 for CE validation
Conclusion
Successful 2026 laser procurement from China requires treating regulatory compliance as the foundational commercial term. Prioritize suppliers with validated certifications, flexible MOQ structures aligned with your market entry strategy, and DDP shipping capabilities to navigate tariff complexities. Partners like Shanghai Carejoy demonstrate how 19+ years of specialized manufacturing expertise translates to reduced audit risk and optimized landed costs. Always conduct pre-shipment inspections per ISO 2859-1 standards to validate laser performance specifications.
Disclaimer: This guide reflects 2026 regulatory landscapes. Verify all requirements with your national competent authority. Shanghai Carejoy is presented as an exemplar of compliant manufacturing – not an exclusive endorsement.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Topic: Frequently Asked Questions – Dental Laser Equipment Procurement (2026 Edition)
Top 5 FAQs for Buying Dental Laser Equipment in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental laser system for my clinic in 2026? | Dental laser systems typically require a stable 110–120V or 220–240V power supply, depending on regional standards and device class. In 2026, many advanced diode and Er:YAG lasers are dual-voltage compatible (auto-switching), but it’s critical to confirm the exact input voltage, frequency (50/60 Hz), and amperage with the manufacturer. Ensure your clinic’s electrical infrastructure includes dedicated circuits and surge protection, especially for high-power systems (e.g., surgical or periodontal lasers). Always consult a certified electrician and review the IEC 60601-1 safety compliance documentation prior to installation. |
| 2. How accessible are spare parts for dental lasers, and what components commonly require replacement? | Spare parts availability varies by manufacturer and model. In 2026, leading brands (e.g., Fotona, Biolase, AMD Lasers) maintain global distribution networks for critical components. Frequently replaced parts include handpieces, fiber tips (for diode lasers), cooling fans, footswitches, and batteries for portable units. When purchasing, confirm the manufacturer’s minimum spare parts availability commitment (ideally 7–10 years post-discontinuation) and whether parts are regionally stocked. Distributors should verify local inventory and lead times. Consider service contracts that include priority access to high-wear components. |
| 3. What does the installation process for a new dental laser involve, and is professional setup required? | Yes, professional installation is mandatory for all Class 3B and Class 4 dental lasers in 2026. The process includes site evaluation (power, ventilation, safety zones), physical setup, calibration, software configuration, and integration with practice management systems (where applicable). Certified biomedical engineers or manufacturer-trained technicians perform installation, ensuring compliance with local laser safety regulations (e.g., ANSI Z136.3 in the U.S.). Clinics must also designate a Laser Safety Officer (LSO) and complete facility risk assessments. Installation typically takes 2–4 hours and is often included in premium purchase packages. |
| 4. What is the standard warranty coverage for dental laser equipment in 2026, and what does it include? | Most reputable manufacturers offer a standard 2-year comprehensive warranty covering parts, labor, and optical components. Extended warranties (up to 5 years) are available for purchase. Coverage typically includes defects in materials and workmanship, laser source longevity, and control system failures. Exclusions usually involve damage from improper use, lack of maintenance, or unauthorized repairs. In 2026, some premium models offer “uptime guarantees” with loaner units during repairs. Distributors should provide detailed warranty terms, service-level agreements (SLA), and clarify international transferability for multi-location practices. |
| 5. Are software updates and calibration covered under warranty or service agreements? | Software updates are generally provided free of charge during the warranty period and are essential for compliance, performance, and new feature rollouts. Remote or on-site updates are included in most service contracts. Calibration—critical for laser accuracy and safety—is typically covered annually under comprehensive maintenance agreements. While basic warranty may not include routine calibration, leading manufacturers bundle it with extended service packages. Confirm update frequency, cybersecurity compliance (e.g., HIPAA, GDPR), and remote diagnostics capability when evaluating systems. |
Need a Quote for Dental Laser Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


